Airborne metal nanoparticles released by azides detonation: determination and potential public exposure
- PMID: 39068190
- PMCID: PMC11283547
- DOI: 10.1038/s41598-024-67540-6
Airborne metal nanoparticles released by azides detonation: determination and potential public exposure
Abstract
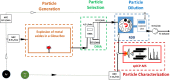
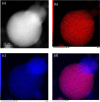
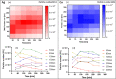
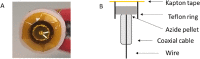
Metal azides are highly energetic materials that release a large amount of gas upon detonation. They also release metal particles, generating an aerosol. The most common azide is sodium azide (NaN3), which is used nowadays in car airbags. If the decomposition is not complete, harmful azide particles might be inhaled. Heavy metal azides find application as a primary explosive (primer) in ammunition. Public health officials have raised concerns about heavy metal particles released during training in shooting ranges. We identify a lack of knowledge on airborne metal particles properties released from azide detonation and on the analytical methods applied to characterize them. As a case study, we detonated milligram amounts of silver azide, copper azide, and a mixture of them in a glove box. We then analyse the airborne particles with an ensemble analytical setup, able to measure real-time their particle size distribution and chemical composition. We detected spherical metal nanoparticles in the range of 2-500 nm. These findings and the developed analytical tools may allow identifying airborne nanoparticles the passenger compartments of vehicles after airbag activation as well as in indoor shooting ranges, contributing to the evaluation of public health risks.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Lathika, A. S., Paramashivan, S. S., Ramasamy, B. K. & Mahadevan, S. Impact of fuel/oxidizer ratio of NaN3 and KNO3 airbag gas generants on toxic emission and performance. Process Saf. Environ. Prot.133, 348–357 (2020).10.1016/j.psep.2019.11.015 - DOI
-
- Wu, T. et al. New coordination complexes-based gas-generating energetic composites. Combust. Flame219, 478–487 (2020).10.1016/j.combustflame.2020.05.022 - DOI
-
- Struve, G. Über das Benzoylhydrazin und einige Derivate desselben. (1891).
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

