Impact of on-trial IGRT quality assurance in an international adaptive radiotherapy trial for participants with bladder cancer
- PMID: 39069085
- PMCID: PMC11413485
- DOI: 10.1016/j.radonc.2024.110460
Impact of on-trial IGRT quality assurance in an international adaptive radiotherapy trial for participants with bladder cancer
Abstract
Background and purpose: Radiotherapy trial quality assurance (RT QA) is crucial for ensuring the safe and reliable delivery of radiotherapy trials, and minimizing inter-institutional variations. While previous studies focused on outlining and planning quality assurance (QA), this work explores the process of Image-Guided Radiotherapy (IGRT), and adaptive radiotherapy. This study presents findings from during-accrual QA in the RAIDER trial, evaluating concordance between online and offline plan selections for bladder cancer participants undergoing adaptive radiotherapy. RAIDER had two seamless stages; stage 1 assessed adherence to dose constraints of dose escalated radiotherapy (DART) and stage 2 assessed safety. The RT QA programme was updated from stage 1 to stage 2.
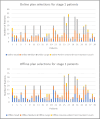
Materials and methods: Data from all participants in the adaptive arms (standard dose adaptive radiotherapy (SART) and DART) of the trial was requested (33 centres across the UK, Australia and New Zealand). Data collection spanned September 2015 to December 2022 and included the plans selected online, on Cone-Beam Computed Tomography (CBCT) data. Concordance with the plans selected offline by the independent RT QA central reviewer was evaluated.
Results: Analysable data was received for 72 participants, giving a total of 884 CBCTs. The overall concordance rate was 83% (723/884). From stage 1 to stage 2 the concordance in the plans selected improved from 75% (369/495) to 91% (354/389).
Conclusion: During-accrual IGRT QA positively influenced plan selection concordance, highlighting the need for ongoing support when introducing a new technique. Overall, it contributes to advancing the understanding and implementation of QA measures in adaptive radiotherapy trials.
Keywords: Adaptive; Bladder cancer; IGRT; Plan selection; Quality assurance.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Robert Huddart reports grants received by their institution from Cancer Research UK, MSD and Roche, consultation fees from Janssen, Bristol Myers Squibb, Astellas, Roche and Nektar Pharmaceuticals, payment or honoraria from Roche, Bristol Myers Squibb and Merck, support for attending meetings and/or travel from MSD, Roche and Janssen, participation on a data safety monitoring board or advisory board for Biontech, Gilead and Merck and leadership or fiduciary role at Cancer Centre London, Parkside. Shaista Hafeez reports grants from NIHR Biomedical Research Centre at The Royal Marsden NHS Foundation Trust and The Institute of Cancer Research, non-financial support from Elekta (Stockholm, Sweden), personal fees and non-financial support from Roche, non-financial support from Merck Sharp & Dohme (MSD), outside the submitted work. EH reports non-financial support (study drug supplies) received by their institution from Astra-Zeneca and Bayer, outside the submitted work. EH also reports grants received by their institution from Cancer Research UK (within scope of submitted work and outside the submitted work) and Prostate Cancer UK (outside submitted work). Amanda Webster, Michael Francis, Hannah Gribble, Clare Griffin, Vibeke N Hansen, Rebecca Lewis, Helen McNair and Elizabeth Miles have no conflicts to declare
Figures
References
-
- RTTQA. About RTTQA RTTQA Group Vision Statement. 2023 [cited 2023 26/06/2023]; Available from: https://rttrialsqa.org.uk/?page_id=14.
-
- EORTC. RADIOTHERAPY RTQA. 2022; Available from: https://www.eortc.org/quality-assurance/rtqa/.
-
- Duhmke E., et al. Low-Dose Radiation Is Sufficient for the Noninvolved Extended-Field Treatment in Favorable Early-Stage Hodgkin’s Disease: Long-Term Results of a Randomized Trial of Radiotherapy Alone. J Clin Oncol. 2001;19:2905–2914. - PubMed
-
- Peters L.J., et al. Critical Impact of Radiotherapy Protocol Compliance and Quality in the Treatment of Advanced Head and Neck Cancer: Results From TROG 02.02. J Clin Oncol. 2010;28:2996–3001. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical