Protein Tyrosine Phosphatase regulation by Reactive Oxygen Species
- PMID: 39069369
- PMCID: PMC12316472
- DOI: 10.1016/bs.acr.2024.05.002
Protein Tyrosine Phosphatase regulation by Reactive Oxygen Species
Abstract
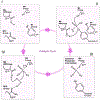
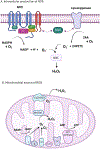
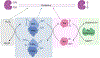
Protein Tyrosine Phosphatases (PTPs) help to maintain the balance of protein phosphorylation signals that drive cell division, proliferation, and differentiation. These enzymes are also well-suited to redox-dependent signaling and oxidative stress response due to their cysteine-based catalytic mechanism, which requires a deprotonated thiol group at the active site. This review focuses on PTP structural characteristics, active site chemical properties, and vulnerability to change by reactive oxygen species (ROS). PTPs can be oxidized and inactivated by H2O2 through three non-exclusive mechanisms. These pathways are dependent on the coordinated actions of other H2O2-sensitive proteins, such as peroxidases like Peroxiredoxins (Prx) and Thioredoxins (Trx). PTPs undergo reversible oxidation by converting their active site cysteine from thiol to sulfenic acid. This sulfenic acid can then react with adjacent cysteines to form disulfide bonds or with nearby amides to form sulfenyl-amide linkages. Further oxidation of the sulfenic acid form to the sulfonic or sulfinic acid forms causes irreversible deactivation. Understanding the structural changes involved in both reversible and irreversible PTP oxidation can help with their chemical manipulation for therapeutic intervention. Nonetheless, more information remains unidentified than is presently known about the precise dynamics of proteins participating in oxidation events, as well as the specific oxidation states that can be targeted for PTPs. This review summarizes current information on PTP-specific oxidation patterns and explains how ROS-mediated signal transmission interacts with phosphorylation-based signaling machinery controlled by growth factor receptors and PTPs.
Keywords: Cysteine; Oxidation; PTP; PTP1B; Phosphatase inactivation; Protein tyrosine phosphatase; ROS; Reactive oxygen species; SHP2.
Copyright © 2024. Published by Elsevier Inc.
Conflict of interest statement
Disclosure of potential conflicts of interest
No potential conflicts of interest are disclosed by the authors.
Figures

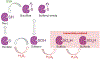


Similar articles
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Redox regulation of protein tyrosine phosphatases: structural and chemical aspects.Antioxid Redox Signal. 2011 Jul 1;15(1):77-97. doi: 10.1089/ars.2010.3611. Epub 2011 Apr 13. Antioxid Redox Signal. 2011. PMID: 20919935 Review.
Cited by
-
Evaluation of the Use of Cell Lines in Studies of Selenium-Dependent Glutathione Peroxidase 2 (GPX2) Involvement in Colorectal Cancer.Diseases. 2024 Sep 10;12(9):207. doi: 10.3390/diseases12090207. Diseases. 2024. PMID: 39329876 Free PMC article.
-
Three STEPs Forward: A Trio of Unexpected Structures of PTPN5.Proteins. 2025 Jul 5:10.1002/prot.70013. doi: 10.1002/prot.70013. Online ahead of print. Proteins. 2025. PMID: 40616465
-
Three STEPs forward: A trio of unexpected structures of PTPN5.bioRxiv [Preprint]. 2025 Feb 18:2024.11.20.624168. doi: 10.1101/2024.11.20.624168. bioRxiv. 2025. Update in: Proteins. 2025 Jul 5. doi: 10.1002/prot.70013. PMID: 39605455 Free PMC article. Updated. Preprint.
-
Reactive oxygen species and oxidative stress in acute pancreatitis: Pathogenesis and new therapeutic interventions.World J Gastroenterol. 2024 Dec 7;30(45):4771-4780. doi: 10.3748/wjg.v30.i45.4771. World J Gastroenterol. 2024. PMID: 39649547 Free PMC article.
-
A functional map of phosphoprotein phosphatase regulation identifies an evolutionary conserved reductase for the catalytic metal ions.bioRxiv [Preprint]. 2025 Feb 16:2025.02.12.637884. doi: 10.1101/2025.02.12.637884. bioRxiv. 2025. PMID: 39990307 Free PMC article. Preprint.
References
-
- Ahuja LG (2018). Protein Tyrosine Phosphatases : Structure, Signaling and Drug Discovery / Lalima G. Ahuja. Berlin: ;: De Gruyter.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

