Peroxisomal cholesterol metabolism regulates yap-signaling, which maintains intestinal epithelial barrier function and is altered in Crohn's disease
- PMID: 39069546
- PMCID: PMC11284232
- DOI: 10.1038/s41419-024-06925-x
Peroxisomal cholesterol metabolism regulates yap-signaling, which maintains intestinal epithelial barrier function and is altered in Crohn's disease
Abstract
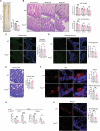
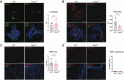
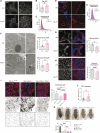
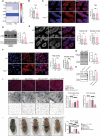
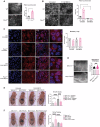
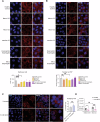
Intestinal epithelial cells line the luminal surface to establish the intestinal barrier, where the cells play essential roles in the digestion of food, absorption of nutrients and water, protection from microbial infections, and maintaining symbiotic interactions with the commensal microbial populations. Maintaining and coordinating all these functions requires tight regulatory signaling, which is essential for intestinal homeostasis and organismal health. Dysfunction of intestinal epithelial cells, indeed, is linked to gastrointestinal disorders such as irritable bowel syndrome, inflammatory bowel disease, and gluten-related enteropathies. Emerging evidence suggests that peroxisome metabolic functions are crucial in maintaining intestinal epithelial cell functions and intestinal epithelium regeneration and, therefore, homeostasis. Here, we investigated the molecular mechanisms by which peroxisome metabolism impacts enteric health using the fruit fly Drosophila melanogaster and murine model organisms and clinical samples. We show that peroxisomes control cellular cholesterol, which in turn regulates the conserved yes-associated protein-signaling and contributes to intestinal epithelial structure and epithelial barrier function. Moreover, analysis of intestinal organoid cultures derived from biopsies of patients affected by Crohn's Disease revealed that the dysregulation of peroxisome number, excessive cellular cholesterol, and inhibition of Yap-signaling are markers of disease and could be novel diagnostic and/or therapeutic targets for treating Crohn's Disease. Our studies provided mechanistic insights on peroxisomal signaling in intestinal epithelial cell functions and identified cholesterol as a novel metabolic regulator of yes-associated protein-signaling in tissue homeostasis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
MeSH terms
Substances
Grants and funding
- RGPIN-2019-04083/Canadian Network for Research and Innovation in Machining Technology, Natural Sciences and Engineering Research Council of Canada (NSERC Canadian Network for Research and Innovation in Machining Technology)
- RGPIN-2019-04083/Canadian Network for Research and Innovation in Machining Technology, Natural Sciences and Engineering Research Council of Canada (NSERC Canadian Network for Research and Innovation in Machining Technology)
- PJT-169179/Gouvernement du Canada | Instituts de Recherche en Santé du Canada | CIHR Skin Research Training Centre (Skin Research Training Centre)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

