Optimization of fluorinated phenyl azides as universal photocrosslinkers for semiconducting polymers
- PMID: 39069548
- PMCID: PMC11284223
- DOI: 10.1038/s41467-024-50257-5
Optimization of fluorinated phenyl azides as universal photocrosslinkers for semiconducting polymers
Abstract
Fluorinated phenyl azides (FPA) enable photo-structuring of π-conjugated polymer films for electronic device applications. Despite their potential, FPAs have faced limitations regarding their crosslinking efficiency, and more importantly, their impact on critical semiconductor properties, such as charge-carrier mobility. Here, we report that azide photolysis and photocrosslinking can achieve unity quantum efficiencies for specific FPAs. This suggests preferential nitrene insertion into unactivated C‒H bonds over benzazirine and ketenimine reactions, which we attribute to rapid interconversion between the initially formed hot states. Furthermore, we establish a structure‒activity relationship for carrier mobility quenching. The binding affinity of FPA crosslinker to polymer π-stacks governs its propensity for mobility quenching in both PM6 and PBDB-T used as model conjugated polymers. This binding affinity can be suppressed by FPA ring substitution, but varies in a non-trivial way with π-stack order. Utilizing the optimal FPA, photocrosslinking enables the fabrication of morphology-stabilized, acceptor-infiltrated donor polymer networks (that is, PBDB-T: ITIC and PM6: Y6) for solar cells. Our findings demonstrate the exceptional potential of the FPA photochemistry and offer a promising approach to address the challenges of modelling realistic molecular interactions in complex polymer morphologies, moving beyond the limitations of Flory‒Huggins mean field theory.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


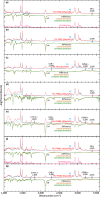





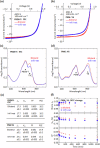

References
-
- Jang, W. et al. Tetrabranched photo-crosslinker enables micrometer-scale patterning of light-emitting Super Yellow for high-resolution OLEDs. ACS Photonics8, 2519–2528 (2021). 10.1021/acsphotonics.1c00768 - DOI
-
- Hengge, M. et al. Crosslinking super yellow to produce super OLEDs: crosslinking with azides enables improved performance. J. Polym. Sci.60, 1878–1886 (2022). 10.1002/pol.20220120 - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

