Characterization of human aquaporin ion channels in a yeast expression system as a tool for novel ion channel discovery
- PMID: 39069912
- PMCID: PMC11358751
- DOI: 10.1042/BSR20240542
Characterization of human aquaporin ion channels in a yeast expression system as a tool for novel ion channel discovery
Abstract
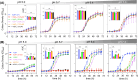
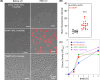
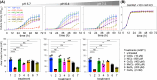
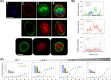
Aquaporin (AQP) channels found in all domains of life are transmembrane proteins which mediate passive transport of water, glycerol, signaling molecules, metabolites, and charged solutes. Discovery of new classes of ion-conducting AQP channels has been slow, likely reflecting time- and labor-intensive methods required for traditional electrophysiology. Work here defines a sensitive mass-throughput system for detecting AQP ion channels, identified by rescue of cell growth in the K+-transport-defective yeast strain CY162 following genetic complementation with heterologously expressed cation-permeable channels, using the well characterized human AQP1 channel for proof of concept. Results showed AQP1 conferred transmembrane permeability to cations which rescued survival in CY162 yeast. Comprehensive testing showed that growth response properties fully recapitulated AQP1 pharmacological agonist and antagonist profiles for activation, inhibition, dose-dependence, and structure-function relationships, demonstrating validity of the yeast screening tool for AQP channel identification and drug discovery efforts. This method also provided new information on divalent cation blockers of AQP1, pH sensitivity of antagonists, and ion permeability of human AQP6. Site-directed mutagenesis of AQP1 channel regulatory domains confirmed that yeast growth rescue was mediated by the introduced channels. Optical monitoring with a lithium-sensitive photoswitchable probe in living cells independently demonstrated monovalent cation permeability of AQP1 channels in yeast plasma membrane. Ion channel properties of AQP1 expressed in yeast were consistent with those of AQP1 expressed in Xenopus laevis oocyte and K+-transport defective Escherichia coli. Outcomes here establish a powerful new approach for efficient screening of phylogenetically diverse AQPs for yet untested functions as cation channels.
Keywords: Saccharomyces cerevisiae; aquaporins; drug discovery and design; high-throughput screening; ion channels.
© 2024 The Author(s).
Conflict of interest statement
The authors declare no conflict of interest; the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Point mutations in the aromatic/arginine region in aquaporin 1 allow passage of urea, glycerol, ammonia, and protons.Proc Natl Acad Sci U S A. 2006 Jan 10;103(2):269-74. doi: 10.1073/pnas.0507225103. Epub 2006 Jan 3. Proc Natl Acad Sci U S A. 2006. PMID: 16407156 Free PMC article.
-
The arginine-facing amino acid residue of the rat aquaporin 1 constriction determines solute selectivity according to its size and lipophilicity.Mol Membr Biol. 2014 Nov-Dec;31(7-8):228-38. doi: 10.3109/09687688.2014.960493. Epub 2014 Oct 24. Mol Membr Biol. 2014. PMID: 25341953
-
Dominant-negative suppression of big brain ion channel activity by mutation of a conserved glutamate in the first transmembrane domain.Gene Expr. 2007;13(6):329-37. doi: 10.3727/000000006781510688. Gene Expr. 2007. PMID: 17708419 Free PMC article.
-
A fascinating tail: cGMP activation of aquaporin-1 ion channels.Trends Pharmacol Sci. 2002 Dec;23(12):558-62. doi: 10.1016/s0165-6147(02)02112-0. Trends Pharmacol Sci. 2002. PMID: 12457773 Review.
-
Functional domains of aquaporin-1: keys to physiology, and targets for drug discovery.Curr Pharm Des. 2007;13(31):3212-21. doi: 10.2174/138161207782341349. Curr Pharm Des. 2007. PMID: 18045170 Review.
Cited by
-
Post-translational modification acts as a digital like switch influencing AtPIP2;1 water and cation permeability.Sci Rep. 2025 Jul 2;15(1):22552. doi: 10.1038/s41598-025-06200-9. Sci Rep. 2025. PMID: 40594625 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

