Rational Design of Far-Red Archaerhodopsin-3-Based Fluorescent Genetically Encoded Voltage Indicators: from Elucidation of the Fluorescence Mechanism in Archers to Novel Red-Shifted Variants
- PMID: 39069984
- PMCID: PMC11274289
- DOI: 10.1021/acsphyschemau.3c00073
Rational Design of Far-Red Archaerhodopsin-3-Based Fluorescent Genetically Encoded Voltage Indicators: from Elucidation of the Fluorescence Mechanism in Archers to Novel Red-Shifted Variants
Abstract
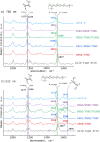
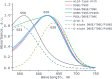
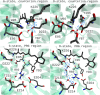
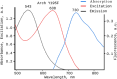
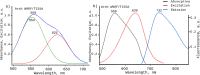
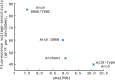
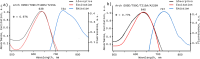
Genetically encoded voltage indicators (GEVIs) have found wide applications as molecular tools for visualization of changes in cell membrane potential. Among others, several classes of archaerhodopsin-3-based GEVIs have been developed and have proved themselves promising in various molecular imaging studies. To expand the application range for this type of GEVIs, new variants with absorption band maxima shifted toward the first biological window and enhanced fluorescence signal are required. Here, we integrate computational and experimental strategies to reveal structural factors that distinguish far-red bright archaerhodopsin-3-based GEVIs, Archers, obtained by directed evolution in a previous study (McIsaac et al., PNAS, 2014) and the wild-type archaerhodopsin-3 with an extremely dim fluorescence signal, aiming to use the obtained information in subsequent rational design. We found that the fluorescence can be enhanced by stabilization of a certain conformation of the protein, which, in turn, can be achieved by tuning the pK a value of two titratable residues. These findings were supported further by introducing mutations into wild-type archeorhodopsin-3 and detecting the enhancement of the fluorescence signal. Finally, we came up with a rational design and proposed previously unknown Archers variants with red-shifted absorption bands (λmax up to 640 nm) and potential-dependent bright fluorescence (quantum yield up to 0.97%).
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Azimi Hashemi N.; Bergs A. C.; Schüler C.; Scheiwe A. R.; Steuer Costa W.; Bach M.; Liewald J. F.; Gottschalk A. Rhodopsin-based voltage imaging tools for use in muscles and neurons of Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 2019, 116, 17051–17060. 10.1073/pnas.1902443116. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
