Advances in targeting LDL cholesterol: PCSK9 inhibitors and beyond
- PMID: 39070027
- PMCID: PMC11278114
- DOI: 10.1016/j.ajpc.2024.100701
Advances in targeting LDL cholesterol: PCSK9 inhibitors and beyond
Abstract
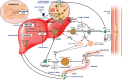
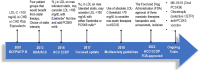
There is a direct relationship between the duration and level of exposure to low density lipoprotein cholesterol (LDL-C) levels over one's lifespan and cardiovascular events. Early treatment to lower elevated LDL-C is crucial for better outcomes with multiple therapies currently available to reduce atherogenic lipoproteins. Statins remain the foundation of LDL-C lowering therapy as one of the most cost-effective drugs to reduce atherosclerotic events (ASCVD) and mortality. Nonetheless, LDL-driven goal attainment remains suboptimal globally, highlighting a considerable need for non-statin therapies to address residual risk related to statin intolerance, non-adherence, and inherited lipoprotein disorders. LDL-C lowering interventions beyond statins include ezetimibe, PCSK9 monoclonal antibodies, inclisiran and bempedoic acid with specific guideline recommendations as to when to consider each. For patients with homozygous familial hypercholesterolemia requiring more advanced therapy, lomitapide and evinacumab are available, providing mechanisms that are not LDL receptor dependent. Lipoprotein apheresis remains an effective option for clinical familial hypercholesterolemia as well as elevated lipoprotein (a). There are investigational therapies being explored to add to our current armamentarium including CETP inhibitors, a third-generation PCSK9 inhibitor (small recombinant fusion protein oral PCSK9 inhibitor) and gene editing which aims to directly restore or disrupt genes of interest at the DNA level. This article is a brief review of the pharmacotherapy options beyond statins for lowering LDL-C and their impact on ASCVD risk reduction. Our primary aim is to guide physicians on the role these therapies play in achieving appropriate LDL-C goals, with an algorithm of when to consider each based on efficacy, safety and outcomes.
Keywords: Advanced therapies; Lipid-lowering; Non-statins; Precision lipidology.
© 2024 The Authors. Published by Elsevier B.V.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Eugenia Gianos reports a relationship with Kaneka Corporation that includes: speaking and lecture fees. Benjamin Hirsh reports a relationship with Amarin Pharma Inc that includes: consulting or advisory. Benjamin Hirsh reports a relationship with Kaneka Corporation that includes: consulting or advisory. Guy Mintz reports a relationship with Novartis Pharmaceuticals Corporation that includes: consulting or advisory and speaking and lecture fees. Guy Mintz reports a relationship with Kaneka Corporation that includes: consulting or advisory. Michael Shapiro reports a relationship with 89bio Inc that includes: funding grants. Michael Shapiro reports a relationship with Esperion Therapeutics Inc that includes: funding grants. Michael Shapiro reports a relationship with Novartis Pharmaceuticals Corporation that includes: board membership, consulting or advisory, and funding grants. Michael Shapiro reports a relationship with Ionis Pharmaceuticals Inc that includes: board membership, consulting or advisory, and funding grants. Michael Shapiro reports a relationship with Merck & Co Inc that includes: funding grants. Michael Shapiro reports a relationship with New Amersterdam that includes: board membership and funding grants. Michael Shapiro reports a relationship with Amgen Inc that includes: board membership, consulting or advisory, and funding grants. Michael Shapiro reports a relationship with Regeneron Pharmaceuticals Inc that includes: consulting or advisory and funding grants. Michael Shapiro reports a relationship with Novo Nordisk Inc that includes: board membership and consulting or advisory. Michael Shapiro reports a relationship with Aidoc that includes: consulting or advisory. Michael Shapiro reports a relationship with Shanghai Pharma Biotherapeutics that includes: consulting or advisory. Michael Shapiro reports a relationship with Emendo Biotherapeutics that includes: consulting or advisory. Michael Shapiro reports a relationship with Kaneka Corporation that includes: consulting or advisory. Michael Shapiro reports a relationship with Agepha that includes: board membership. Michael Shapiro reports a relationship with Precision Biosciences Inc that includes: board membership. Michael Shapiro reports a relationship with American Society for Preventive Cardiology that includes: board membership. Daniel Soffer reports a relationship with Ionis Pharmaceuticals Inc that includes: consulting or advisory. Daniel Soffer reports a relationship with Novartis Pharmaceuticals Corporation that includes: consulting or advisory. Daniel Soffer reports a relationship with Amgen Inc that includes: board membership and consulting or advisory. Daniel Soffer reports a relationship with Amryt Pharmaceuticals Inc that includes: consulting or advisory. Daniel Soffer reports a relationship with GeninCode that includes: consulting or advisory. Daniel Soffer reports a relationship with PHAR that includes: consulting or advisory. Daniel Soffer reports a relationship with Endless Health that includes: consulting or advisory. Daniel Soffer reports a relationship with National Lipid Association that includes: board membership. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Domanski M.J., Tian X., Wu C.O., et al. Time course of LDL cholesterol exposure and cardiovascular disease event risk. J Am Coll Cardiol. 2020;76:1507–1516. - PubMed
-
- Martin S.S., Aday A.W., Almarzooq Z.I., et al. 2024 heart disease and stroke statistics: a report of us and global data from the american heart association. Circulation. 2024;149:e347–e913. - PubMed
-
- Fulcher J., O'Connell R., Voysey M., et al. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385:1397–1405. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

