Similar Limited Protection Against Severe Acute Respiratory Syndrome Coronavirus 2 Omicron Infection in Vaccinated Individuals With HIV and Comparable Controls
- PMID: 39070044
- PMCID: PMC11273239
- DOI: 10.1093/ofid/ofae380
Similar Limited Protection Against Severe Acute Respiratory Syndrome Coronavirus 2 Omicron Infection in Vaccinated Individuals With HIV and Comparable Controls
Abstract
Background: Little is known about the risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron infection in people with human immunodeficiency virus (HIV; PWH) with vaccine-induced or hybrid immunity. We assessed the incidence of Omicron infection in 209 AGEhIV coronavirus disease 2019 substudy participants with well-controlled HIV on antiretroviral therapy and 280 comparable controls, who had received at least the primary vaccination series.
Methods: From September 2020 onward, participants were assessed every 6 months for the incidence of SARS-CoV-2 infection, per SARS-CoV-2 nucleocapsid antibody assay or self-reported positive antigen or polymerase chain reaction test. Between 1 January and 31 October 2022, the cumulative incidence of Omicron infection and associated risk factors were estimated using a conditional risk-set Cox proportional hazards model.
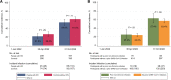
Results: The cumulative incidence of a first Omicron infection was 58.3% by 31 October 2022, not significantly different between groups. HIV status was not independently associated with acquiring Omicron infection. Former and current smoking, as well as an increased predicted anti-spike immunoglobulin G titer were significantly associated with a lower risk of Omicron infection. The majority of infections were symptomatic, but none required hospitalization.
Conclusions: People with well-controlled HIV and controls in our cohort experienced a similarly high proportion of Omicron infections. More booster vaccinations significantly reduced the risk of infection. Clinical Trial Registration. NCT01466582.
Keywords: HIV; Omicron variant; SARS-CoV-2; incidence; serology.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. A. B. has received speaker fees from Gilead Sciences. M. F. S. v. d. L. has received independent scientific grant support from GSK, Sanofi Pasteur, MSD Janssen Infectious Diseases and Vaccines, and Merck & Co, all paid to his institution; has served on advisory boards for GlaxoSmithKline, Novosanis, and Merck & Co; and has received nonfinancial support from Stichting Pathologie Onderzoek en Ontwikkeling. F. W. N. M. W. has served on scientific advisory boards for ViiV Healthcare and Gilead Sciences. M. v. d. V., through his institution, has received independent scientific grant support and consultancy fees from AbbVie, Gilead Sciences, MSD, and ViiV Healthcare, for which honoraria were all paid to his institution. P. R., through his institution, has received independent scientific grant support from Gilead Sciences, Janssen Pharmaceuticals, Merck & Co and ViiV Healthcare and has served on scientific advisory boards for Gilead Sciences, ViiV Healthcare, and Merck & Co, honoraria for which were all paid to his institution. All other authors report no potential conflicts.
Figures
References
-
- National Institute for Public Health and the Environment . Variants of the coronavirus SARS-CoV-2. Available at: https://www.rivm.nl/en/coronavirus-covid-19/virus/variants. Accessed 1 September 2023.
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

