Hyperbaric Oxygen Post Established Stroke
- PMID: 39070389
- PMCID: PMC11283856
- DOI: 10.7759/cureus.63395
Hyperbaric Oxygen Post Established Stroke
Abstract
Background and purpose: Hyperbaric oxygen therapy (HBOT) has been reported to improve neurological function in the chronic phase of stroke in a single trial having significant limitations, including a lack of a sham control.
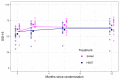
Methods: We conducted a single-center, parallel-group, randomized trial to determine the effectiveness of HBOT compared with a sham control in adults who were 6 to 36 months post-ischemic stroke. The treatment group received 40 sessions of HBOT at the Vancouver General Hospital Hyperbaric Unit. The control group received 40 sessions of sham treatment designed to replicate an HBOT experience. Due to recruitment challenges and timeline/feasibility tracking by the research team, the control arm was altered after 20 months to a waitlist in the hope of increasing participation. In the second phase, participants were randomized to receive HBOT immediately or following an eight-week observation period. The primary outcome was the post-treatment Stroke Impact Scale-16 (SIS-16). Secondary outcomes included the National Institute of Health Stroke Scale, Berg Balance Test, Digit Symbol Substitution Test, 5-Metre Walk Test, 6-Minute Walk Test, Grip Strength, Montreal Cognitive Assessment, Box/Block Test, and Center for Epidemiological Studies - Depression and Short Form-36. Based on detecting a clinically important between-group difference of 10 on the SIS-16 score, our target sample size was 68 participants per arm. Results: From January 5, 2016 to October 9, 2018, 34 participants were enrolled in the trial, 27 during the first phase and seven in the second phase. The study was stopped after 36 months, and prior to meeting the sample size target, due to low recruitment. At the end of treatment, the difference in the SIS-16 between groups was 5.5 (95% CI: 1.3 to 9.7, p = 0.01) in favor of the sham group.
Conclusions: Our results exclude a clinically important benefit of HBOT on the primary outcome of the SIS-16. These findings do not support the use of HBOT in chronic stroke survivors.
Keywords: hyperbaric; hyperbaric oxygen; ischemic stroke; oxygen; rehabilitation; stroke; stroke recovery; stroke rehabilitation.
Copyright © 2024, Harrison et al.
Conflict of interest statement
Human subjects: Consent was obtained or waived by all participants in this study. University of British Columbia Clinical Research Ethics Board issued approval H15-00766. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- Stroke. Donnan GA, Fisher M, Macleod M, Davis SM. Lancet. 2008;371:1612–1623. - PubMed
-
- Cost avoidance associated with optimal stroke care in Canada. Krueger H, Lindsay P, Cote R, Kapral MK, Kaczorowski J, Hill MD. Stroke. 2012;43:2198–2206. - PubMed
-
- Neurologic and functional recovery the Copenhagen Stroke Study. Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS. Phys Med Rehabil Clin N Am. 1999;10:887–906. - PubMed
LinkOut - more resources
Full Text Sources