Sacubitril/Valsartan Improves Cardiac Function in Dialysis Patients
- PMID: 39070454
- PMCID: PMC11283675
- DOI: 10.7759/cureus.63360
Sacubitril/Valsartan Improves Cardiac Function in Dialysis Patients
Abstract
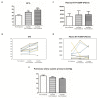
Heart failure (HF) is characterized by the activation of adverse neurohormonal systems and a high mortality rate. Noteworthy, HF is a well-known complication of chronic kidney disease (CKD), especially in end-stage kidney disease (ESKD), where dialysis patients are seven to eight times more likely to encounter cardiac arrest than the general population. Therefore, it is important to develop efficient treatments to improve cardiac function in dialysis patients and eventually reduce the cardiovascular death toll. Sacubitril/valsartan (Sac/Val) is a dual inhibitor/blocker of the neprilysin and angiotensin II receptors, which exert cardioprotective effects among patients with heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved EF (HFpEF). Unfortunately, the drug is not approved for subjects with advanced CKD or dialysis patients due to safety concerns. The current study examined the cardiac effects of Sac/Val in HD patients. Administration of Sac/Val (100-400 mg/day) to 12 hemodialysis (HD) patients with HFrEF for six months gradually improved ejection fraction (EF) independently of morphological changes in cardiac geometry, as assessed by echocardiography (ECHO), and hemodynamic alterations. Interestingly, the Cardiomyopathy Questionnaire (Kansas City KCCQ-12) revealed that quality of life significantly improved after Sac/Val treatment. No major adverse effects were reported in the present study, supporting the safety of Sac/Val at least in these patients and for the applied follow-up period. Collectively, these findings support the use of Sac/Val as a cardioprotective agent in both HD and peritoneal dialysis (PD) patients. Yet, a more comprehensive study is required to establish these findings and to extend the follow-up period for 12 months in order to solidify these encouraging results.
Keywords: dialysis; end-stage renal disease (esrd); heart failure with reduced ejection fraction; heath-related quality of life; sacubitril/valsartan.
Copyright © 2024, Armaly et al.
Conflict of interest statement
Human subjects: Consent was obtained or waived by all participants in this study. Nazareth Hospital EMMS Human Research Review Committee issued approval (22-21-EMMS). This study was conducted in both hemodialysis (HD) and peritoneal dialysis (PD) patients who were prescribed hemolysis and peritoneal dialysis treatment in the Department of Nephrology at Nazareth Hospital, EMMS Nazareth. The study was carried out at Nazareth Hospital. All patients provided informed consent. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures

References
-
- Clinical pharmacokinetics of sacubitril/valsartan (LCZ696): a novel angiotensin receptor-neprilysin inhibitor. Ayalasomayajula S, Langenickel T, Pal P, Boggarapu S, Sunkara G. Clin Pharmacokinet. 2017;56:1461–1478. - PubMed
-
- 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Failure Society of America. Yancy CW, Jessup M, Bozkurt B, et al. J Am Coll Cardiol. 2016;68:1476–1488. - PubMed
-
- 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (esc)developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Ponikowski P, Voors AA, Anker SD, et al. Eur Heart J. 2016;37:2129–2200. - PubMed
-
- Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF) McMurray JJ, Packer M, Desai AS, et al. Eur J Heart Fail. 2013;15:1062–1073. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
