This is a preprint.
Activation of a GPCR, ORL1 receptor: A novel therapy to prevent heart failure progression
- PMID: 39070633
- PMCID: PMC11275996
- DOI: 10.21203/rs.3.rs-4578315/v1
Activation of a GPCR, ORL1 receptor: A novel therapy to prevent heart failure progression
Update in
-
Activation of a GPCR, ORL1 Receptor: A Novel Therapy to Prevent Heart Failure Progression.J Cardiovasc Dev Dis. 2024 Nov 5;11(11):355. doi: 10.3390/jcdd11110355. J Cardiovasc Dev Dis. 2024. PMID: 39590198 Free PMC article.
Abstract
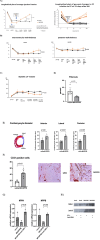
Purpose: The number of ischemic heart failure (HF) patients is growing dramatically worldwide. However, there are at present no preventive treatments for HF. Our previous study showed that Gata4 overexpression improved cardiac function after myocardial infarction in the rat heart. We also found that Gata4 overexpression significantly increased a Pnoc gene expression, an endogenous ligand for cell membrane receptor, ORL1. We hypothesized that an activation of ORL1 receptor would suppress HF in a rat ischemic heart model.
Method: Adult Sprague Dawley rats (8 weeks old, 6 males and 6 females) underwent left anterior descending coronary artery ligation. Three weeks later, normal saline or MCOPPB (ORL1 activator, 2.5mg/kg/day) intraperitoneal injection was started, and continued 5 days a week, for 3 months. Echocardiography was performed six times, pre-operative, 3 days after coronary artery ligation, pre-MCOPPB or saline injection, and 1, 2, and 3 months after saline or MCOPPB injection started. Animals were euthanized after 3 months follow up and the heart was harvested for histological analysis.
Results: ORL1 activator, MCOPPB, significantly improved cardiac function after myocardial infarction in rat (Ejection fraction, MCOPPB vs saline at euthanasia, 67 ± 3 vs 43 ± 2, p < 0.001). MCOPPB also decreased fibrosis and induced angiogenesis.
Conclusion: ORL1 activator, MCOPPB, may be a novel treatment for preventing HF progression.
Keywords: MCOPPB; ORL1 receptor activation; Prevention of ischemic heart failure progression.
Conflict of interest statement
Declarations Conflicts of interest/Competing interests: none
Figures

References
-
- Mathison M, Singh VP, Sanagasetti D, Yang L, Pinnamaneni JP, Yang J, Rosengart TK. Cardiac reprogramming factor Gata4 reduces postinfarct cardiac fibrosis through direct repression of the profibrotic mediator snail. J Thorac Cardiovasc Surg. 2017;154:1601–1610. DOI: 10.1016/j.jtcvs.2017.06.035. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

