This is a preprint.
Biomolecular condensates can enhance pathological RNA clustering
- PMID: 39070659
- PMCID: PMC11276000
- DOI: 10.21203/rs.3.rs-4557520/v1
Biomolecular condensates can enhance pathological RNA clustering
Update in
-
Homotypic RNA clustering accompanies a liquid-to-solid transition inside the core of multi-component biomolecular condensates.Nat Chem. 2025 Aug;17(8):1236-1246. doi: 10.1038/s41557-025-01847-3. Epub 2025 Jul 2. Nat Chem. 2025. PMID: 40603603
Abstract
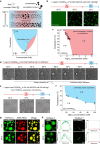
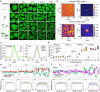
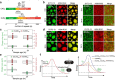
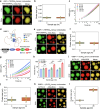
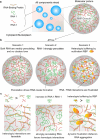
Intracellular aggregation of repeat expanded RNA has been implicated in many neurological disorders. Here, we study the role of biomolecular condensates on irreversible RNA clustering. We find that physiologically relevant and disease-associated repeat RNAs spontaneously undergo an age-dependent percolation transition inside multi-component protein-nucleic acid condensates to form nanoscale clusters. Homotypic RNA clusters drive the emergence of multiphasic condensate structures with an RNA-rich solid core surrounded by an RNA-depleted fluid shell. The timescale of the RNA clustering, which drives a liquid-to-solid transition of biomolecular condensates, is determined by the sequence features, stability of RNA secondary structure, and repeat length. Importantly, G3BP1, the core scaffold of stress granules, introduces heterotypic buffering to homotypic RNA-RNA interactions and impedes intra-condensate RNA clustering in an ATP-independent manner. Our work suggests that biomolecular condensates can act as sites for RNA aggregation. It also highlights the functional role of RNA-binding proteins in suppressing aberrant RNA phase transitions.
Conflict of interest statement
Competing interests P.R.B. is a member of the Biophysics Reviews (AIP Publishing) editorial board. This affiliation did not influence the work reported here. All other authors have no conflicts to report. Additional Declarations: There is NO Competing Interest.
Figures


References
-
- Shin Y, Brangwynne CP. Liquid phase condensation in cell physiology and disease. Science 2017, 357(6357): eaaf4382. - PubMed
-
- Brangwynne CP, Eckmann CR, Courson DS, Rybarska A, Hoege C, Gharakhani J, et al. Germline P Granules Are Liquid Droplets That Localize by Controlled Dissolution/Condensation. Science 2009, 324(5935): 1729–1732. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

