Rolosense: Mechanical Detection of SARS-CoV-2 Using a DNA-Based Motor
- PMID: 39071064
- PMCID: PMC11273449
- DOI: 10.1021/acscentsci.4c00312
Rolosense: Mechanical Detection of SARS-CoV-2 Using a DNA-Based Motor
Abstract
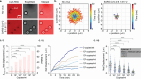
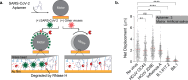
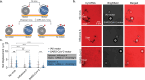
Assays that detect viral infections play a significant role in limiting the spread of diseases such as SARS-CoV-2. Here, we present Rolosense, a virus sensing platform that leverages the motion of 5 μm DNA-based motors on RNA fuel chips to transduce the presence of viruses. Motors and chips are modified with aptamers, which are designed for multivalent binding to viral targets and lead to stalling of motion. Therefore, the motors perform a "mechanical test" of the viral target and stall in the presence of whole virions, which represents a unique mechanism of transduction distinct from conventional assays. Rolosense can detect SARS-CoV-2 spiked in artificial saliva and exhaled breath condensate with a sensitivity of 103 copies/mL and discriminates among other respiratory viruses. The assay is modular and amenable to multiplexing, as demonstrated by our one-pot detection of influenza A and SARS-CoV-2. As a proof of concept, we show that readout can be achieved using a smartphone camera with a microscopic attachment in as little as 15 min without amplification reactions. Taken together, these results show that mechanical detection using Rolosense can be broadly applied to any viral target and has the potential to enable rapid, low-cost point-of-care screening of circulating viruses.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Update of
-
Rolosense: Mechanical detection of SARS-CoV-2 using a DNA-based motor.bioRxiv [Preprint]. 2023 Feb 28:2023.02.27.530294. doi: 10.1101/2023.02.27.530294. bioRxiv. 2023. Update in: ACS Cent Sci. 2024 May 21;10(7):1332-1347. doi: 10.1021/acscentsci.4c00312. PMID: 36909543 Free PMC article. Updated. Preprint.
Similar articles
-
Rolosense: Mechanical detection of SARS-CoV-2 using a DNA-based motor.bioRxiv [Preprint]. 2023 Feb 28:2023.02.27.530294. doi: 10.1101/2023.02.27.530294. bioRxiv. 2023. Update in: ACS Cent Sci. 2024 May 21;10(7):1332-1347. doi: 10.1021/acscentsci.4c00312. PMID: 36909543 Free PMC article. Updated. Preprint.
-
Assessment of a Smartphone-Based Loop-Mediated Isothermal Amplification Assay for Detection of SARS-CoV-2 and Influenza Viruses.JAMA Netw Open. 2022 Jan 4;5(1):e2145669. doi: 10.1001/jamanetworkopen.2021.45669. JAMA Netw Open. 2022. PMID: 35089353 Free PMC article.
-
A mask-based diagnostic platform for point-of-care screening of Covid-19.Biosens Bioelectron. 2021 Nov 15;192:113486. doi: 10.1016/j.bios.2021.113486. Epub 2021 Jul 8. Biosens Bioelectron. 2021. PMID: 34260968 Free PMC article.
-
Heterogeneity in transmissibility and shedding SARS-CoV-2 via droplets and aerosols.Elife. 2021 Apr 16;10:e65774. doi: 10.7554/eLife.65774. Elife. 2021. PMID: 33861198 Free PMC article.
-
Aptamer-based Emerging Tools for Viral Biomarker Detection: A Focus on SARS-CoV-2.Curr Med Chem. 2023;30(8):910-934. doi: 10.2174/1568009622666220214101059. Curr Med Chem. 2023. PMID: 35156569 Review.
Cited by
-
On-Demand Photoactivation of DNA-Based Motor Motion.ACS Nano. 2025 Feb 11;19(5):5363-5375. doi: 10.1021/acsnano.4c13068. Epub 2025 Jan 30. ACS Nano. 2025. PMID: 39883883 Free PMC article.
-
Microparticle-Based Detection of Viruses.Biosensors (Basel). 2023 Aug 15;13(8):820. doi: 10.3390/bios13080820. Biosensors (Basel). 2023. PMID: 37622906 Free PMC article. Review.
-
High-throughput microbead assay system with a portable, cost-effective Wi-Fi imaging module, and disposable multi-layered microfluidic cartridges for virus and microparticle detection, and tracking.Biomed Microdevices. 2023 Jun 7;25(3):21. doi: 10.1007/s10544-023-00661-3. Biomed Microdevices. 2023. PMID: 37284878 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
