Biosimilar underutilization alone does not foretell a broken biologics market
- PMID: 39071106
- PMCID: PMC11282456
- DOI: 10.1093/haschl/qxae090
Biosimilar underutilization alone does not foretell a broken biologics market
Abstract
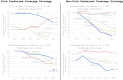
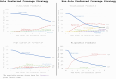
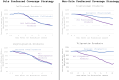
Biosimilars offer the potential for cost savings and expanded access to biologic products; however, there are concerns regarding the rate of biosimilar uptake. We assessed the relationship between biosimilar and originator pricing, coverage, and market share by describing four case studies that fall into two categories: (1) sole preferred coverage strategy (ie, aim is to have originator product preferred; biosimilar(s) non-preferred), defined as steep average sales price (ASP) reductions for originator products (decline in net prices by at least 50% following the introduction of biosimilar competition by 2022) and (2) non-sole preferred coverage strategy (ie, aim is to have originator product preferred alongside biosimilar products), defined as moderate ASP reductions for originator products with (net prices did not decline by at least 50% of its pre-biosimilar competition value). We found that originators with sole preferred coverage strategies maintained formulary preference and market share relative to originators with non-sole preferred coverage strategies. Regardless of strategy, the market-weighted ASP for all four product families (originator and biosimilars) declined significantly in the years following the introduction of biosimilars, suggesting that biosimilar uptake alone may not be a complete measure of whether the biosimilar market is facilitating competition and lowering prices.
Keywords: ASP; WAC; average sales price; biologics; biosimilar competition; biosimilars; filgrastim; infliximab; originator; payer coverage; pegfilgrastim; trastuzumab; wholesale acquisition cost.
© The Author(s) 2024. Published by Oxford University Press on behalf of Project HOPE - The People-To-People Health Foundation, Inc.
Conflict of interest statement
Conflicts of interest Please see ICMJE form(s) for author conflicts of interest. These have been provided as supplementary materials.
Figures
References
-
- Patient Protection and Affordable Care Act, Pub. L. No. 111-148, 42 USC 201 note. 686-703 §7001-7003; 2010. Accessed March 11, 2024. https://www.congress.gov/bill/111th-congress/house-bill/35902.
-
- U.S. Food and Drug Administration . Biosimilars: review and approval. Updated December 13, 2022. Accessed March 11, 2024. https://www.fda.gov/drugs/biosimilars/review-and-approval
-
- Attebery, P, Bach PB, Ohn JA, Trusheim MR. Biologics are natural monopolies (part 1): why biosimilars do not create effective competition. Health Aff Forefront. 2019. Accessed June 5, 2024. 10.1377/hblog20190405.396631 - DOI
LinkOut - more resources
Full Text Sources
