Transcriptional response of the heart to vagus nerve stimulation
- PMID: 39071113
- PMCID: PMC7616044
- DOI: 10.6084/m9.figshare.24449590
Transcriptional response of the heart to vagus nerve stimulation
Abstract
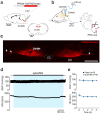
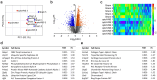
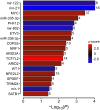
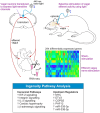
Heart failure is a major clinical problem, with treatments involving medication, devices, and emerging neuromodulation therapies such as vagus nerve stimulation (VNS). Considering the ongoing interest in using VNS to treat cardiovascular disease it is important to understand the genetic and molecular changes developing in the heart in response to this form of autonomic neuromodulation. This experimental animal (rat) study investigated the immediate transcriptional response of the ventricular myocardium to selective stimulation of vagal efferent activity using an optogenetic approach. Vagal preganglionic neurons in the dorsal motor nucleus of the vagus nerve were genetically targeted to express light-sensitive chimeric channelrhodopsin variant ChIEF, and stimulated using light. RNA sequencing of left ventricular myocardium identified 294 differentially expressed genes (DEGs, false discovery rate <0.05). Qiagen Ingenuity Pathway Analysis (IPA) highlighted 118 canonical pathways that were significantly modulated by vagal activity, of which 14 had a z-score of ≥2/≤-2, including EIF-2, IL-2, Integrin, and NFAT-regulated cardiac hypertrophy. IPA revealed the effect of efferent vagus stimulation on protein synthesis, autophagy, fibrosis, autonomic signalling, inflammation, and hypertrophy. IPA further predicted that the identified DEGs were the targets of 50 upstream regulators, including transcription factors (e.g., MYC, NRF1) and microRNAs (e.g., miR-335-3p, miR-338-3p). These data demonstrate that the vagus nerve has a major impact on myocardial expression of genes involved in regulation of key biological pathways. The transcriptional response of the ventricular myocardium induced by stimulation of vagal efferents is consistent with the beneficial effect of maintained/increased vagal activity on the heart.
Keywords: RNA sequencing; autonomic nervous system; heart; transcriptome; vagus nerve..
Conflict of interest statement
Disclosures The authors declare no competing interests.
Figures

References
-
- Fiuzat M, Hamo CE, Butler J, Abraham WT, DeFilippis EM, Fonarow GC, Lindenfeld J, Mentz RJ, Psotka MA, Solomon SD, Teerlink JR, et al. Optimal Background Pharmacological Therapy for Heart Failure Patients in Clinical Trials: JACC Review Topic of the Week. J Am Coll Cardiol. 2022;79:504–510. - PMC - PubMed
-
- Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, Fang JC, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895–e1032. - PubMed
-
- Wang Z, Wu Y, Zhang J. Cardiac resynchronization therapy in heart failure patients: tough road but clear future. Heart Fail Rev. 2021;26:735–745. - PubMed
-
- Cappannoli L, Scacciavillani R, Rocco E, Perna F, Narducci ML, Vaccarella M, D’Amario D, Pelargonio G, Massetti M, Crea F, Aspromonte N. Cardiac contractility modulation for patient with refractory heart failure: an updated evidence-based review. Heart Fail Rev. 2021;26:227–235. - PubMed
-
- Edelson JB, Genuardi MV, Santangeli P, Birati EY. Cardiac Contractility Monitoring: an Important Therapy in the Treatment of Advanced Heart Failure. Curr Cardiol Rep. 2020;22:81. - PubMed
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources

