Screening for anti-influenza virus compounds from traditional Mongolian medicine by GFP-based reporter virus
- PMID: 39071166
- PMCID: PMC11272615
- DOI: 10.3389/fcimb.2024.1431979
Screening for anti-influenza virus compounds from traditional Mongolian medicine by GFP-based reporter virus
Abstract
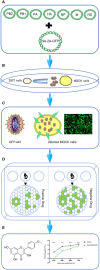
Introduction: Screening for effective antiviral compounds from traditional Mongolian medicine not only aids in the research of antiviral mechanisms of traditional medicines, but is also of significant importance for the development of new antiviral drugs targeting influenza A virus. Our study aimed to establish high-throughput, rapid screening methods for antiviral compounds against influenza A virus from abundant resources of Mongolian medicine.
Methods: The use of GFP-based reporter viruses plays a pivotal role in antiviral drugs screening by enabling rapid and precise identification of compounds that inhibit viral replication. Herein, a GFP-based reporter influenza A virus was used to identify potent anti-influenza compounds within traditional Mongolian medicine.
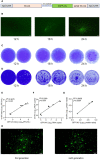
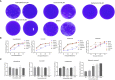
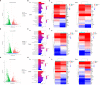
Results: Our study led to the discovery of three active compounds: Cardamonin, Curcumin, and Kaempferide, all of which exhibited significant antiviral properties in vitro. Subsequent analysis confirmed that their effectiveness was largely due to the stimulation of the antiviral signaling pathways of host cells, rather than direct interference with the viral components, such as the viral polymerase.
Discussion: This study showcased the use of GFP-based reporter viruses in high-throughput screening to unearth antiviral agents from traditional Mongolian medicine, which contains rich antiviral compounds and deserves further exploration. Despite certain limitations, fluorescent reporter viruses present substantial potential for antiviral drug screening research due to their high throughput and efficiency.
Keywords: Cardamonin; Curcumin; GFP-based reporter virus; influenza A virus; kaempferide; traditional mongolian medicine.
Copyright © 2024 Nie, Li, Zhang, Zeng, Cai, Peng, Jiang, Shi and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

