This is a preprint.
Computational structural prediction and chemical inhibition of the human mitochondrial pyruvate carrier protein heterodimer complex
- PMID: 39071381
- PMCID: PMC11275797
- DOI: 10.1101/2024.05.16.594520
Computational structural prediction and chemical inhibition of the human mitochondrial pyruvate carrier protein heterodimer complex
Abstract
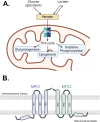
The mitochondrial pyruvate carrier (MPC) plays a role in numerous diseases including neurodegeneration, metabolically dependent cancers, and the development of insulin resistance. Several previous studies in genetic mouse models or with existing inhibitors suggest that inhibition of the MPC could be used as a viable therapeutic strategy in these diseases. However, the MPC's structure is unknown, making it difficult to screen for and develop therapeutically viable inhibitors. Currently known MPC inhibitors would make for poor drugs due to their poor pharmacokinetic properties, or in the case of the thiazolidinediones (TZDs), off-target specificity for peroxisome-proliferator activated receptor gamma (PPARγ) leads to unwanted side effects. In this study, we develop several structural models for the MPC heterodimer complex and investigate the chemical interactions required for the binding of these known inhibitors to MPC and PPARγ. Based on these models, the MPC most likely takes on outward-facing (OF) and inward-facing (IF) conformations during pyruvate transport, and inhibitors likely plug the carrier to inhibit pyruvate transport. Although some chemical interactions are similar between MPC and PPARγ binding, there is likely enough difference to reduce PPARγ specificity for future development of novel, more specific MPC inhibitors.
Keywords: UK-5099; mitochondria; mitochondrial pyruvate carrier; pyruvate transporter; thiazolidinedione.
Conflict of interest statement
Conflicts of Interest Statement None declared.
Figures






References
-
- Nagampalli R. S. K., Quesnay J. E. N., Adamoski D., Islam Z., Birch J., Sebinelli H. G., Girard R., Ascencao C. F. R., Fala A. M., PauleK B. A., Consonni S. R., de Oliveira J. F., Silva A. C. T., Franchini K. G., Leme A. F. P., Silber A. M., Ciancaglini P., Moraes I., Dias S. M. G., Ambrosio A. L. B., Sci Rep 2018, 8, 3510. - PMC - PubMed
-
- Lee J., Jin Z., Lee D., Yun J. H., Lee W., Int J Mol Sci 2020, 21.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
