This is a preprint.
Developmental timecourse of aptitude for motor skill learning in mouse
- PMID: 39071410
- PMCID: PMC11275902
- DOI: 10.1101/2024.07.19.604309
Developmental timecourse of aptitude for motor skill learning in mouse
Abstract
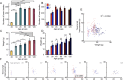
Learning motor skills requires plasticity in the primary motor cortex (M1). But the capacity for cortical circuit plasticity varies over developmental age in sensory cortex. This study assesses the normal developmental trajectory of motor learning to assess how aptitude might vary with age. We trained mice of both sexes to run on a custom accelerating rotarod at ages from postnatal day (P) 20 to P120, tracking paw position and quantifying time to fall and changes in gait pattern. While animals of all ages were able to perform better after five training sessions, performance improved most rapidly on the first training day for mice between ages P30-60, suggesting an age with heightened plasticity. Learning this task required M1, because pharmacological inactivation of M1 prevented improvement in task performance. Paw position and gait patterns changed with learning, though differently between age groups. Successful mice learned to shift their gait from hopping to walking. Notably, this shift in gait happened earlier in the trial for forelimbs in comparison to hindlimbs. Thus, motor plasticity might more readily occur in forelimbs. Changes in gait and other kinematic parameters are an additional learning metric beyond time to fall, offering insight into how mice improve performance. Overall, these results suggest mouse motor learning has a developmental trajectory.
Conflict of interest statement
Conflict of interest statement: The authors declare no competing financial interests.
Figures






References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
