This is a preprint.
Chlamydia muridarum Causes Persistent Subclinical Infection and Elicits Innate and Adaptive Immune Responses in C57BL/6J, BALB/cJ and J:ARC(S) Mice Following Exposure to Shedding Mice
- PMID: 39071441
- PMCID: PMC11275779
- DOI: 10.1101/2024.07.16.603732
Chlamydia muridarum Causes Persistent Subclinical Infection and Elicits Innate and Adaptive Immune Responses in C57BL/6J, BALB/cJ and J:ARC(S) Mice Following Exposure to Shedding Mice
Update in
-
Chlamydia muridarum Causes Persistent Subclinical Infection and Elicits Innate and Adaptive Immune Responses in C57BL/6J, BALB/cJ, and J:ARC(S) Mice Following Exposure to Shedding Mice.Comp Med. 2024 Dec 1;74(6):373-391. doi: 10.30802/AALAS-CM-24-057. Comp Med. 2024. PMID: 39853328 Free PMC article.
Abstract
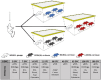
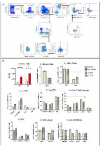
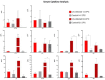
Chlamydia muridarum (Cm) has reemerged as a moderately prevalent infectious agent in research mouse colonies. Despite its' experimental use, few studies evaluate Cm's effects on immunocompetent mice following its natural route of infection. A Cm field isolate was administered (orogastric gavage) to 8-week-old female BALB/cJ (C) mice. After confirming shedding (through 95d), these mice were cohoused with naïve C57BL/6J (B6), C, and Swiss (J:ARC[S]) mice (n=28/strain) for 30 days. Cohoused mice (n=3-6 exposed and 1-6 control/strain) were evaluated 7, 14, 21, 63, 120, and 180 days post-cohousing (DPC) via hemograms, serum biochemistry analysis, fecal qPCR, histopathology, and Cm MOMP immunohistochemistry. Immunophenotyping was performed on spleen (B6, C, S; n=6/strain) and intestines (B6; n=6) at 14 and 63 DPC. Serum cytokine concentrations were measured (B6; n=6 exposed and 2 control) at 14 and 63 DPC. All B6 mice were shedding Cm by 3 through 180 DPI. One of 3 C and 1 of 6 S mice began shedding Cm at 3 and 14 DPC, respectively, with the remaining shedding thereafter. Clinical pathology was nonremarkable. Minimal-to-moderate enterotyphlocolitis and gastrointestinal associated lymphoid tissue (GALT) hyperplasia was observed in 15 and 47 of 76 Cm-infected mice, respectively. Cm antigen was frequently detected in GALT-associated surface intestinal epithelial cells. Splenic immunophenotyping revealed increased monocytes and shifts in T cell population subsets in all strains/timepoints. Gastrointestinal immunophenotyping (B6) revealed sustained increases in total inflammatory cells and elevated cytokine production in innate lymphoid cells and effector T cells (large intestine). Elevated concentrations of pro-inflammatory cytokines were detected in the serum (B6). Results demonstrate that while clinical disease was not appreciated, 3 commonly utilized strains of mice are susceptible to chronic enteric Cm infection which may alter various immune responses. Considering the widespread use of mice to model GI disease, institutions should consider excluding Cm from their colonies.
Conflict of interest statement
Conflicts of Interest Kenneth Henderson, Cheryl Woods, and Panagiota Momtsios are employees of Charles River Laboratories, a company that produces and distributes research models and provides diagnostic services. The other authors have no competing interest to declare.
Figures







References
-
- American Association for Laboratory Animal Services. [Internet]. 2021. Alleviating pain and distress in laboratory animals. [Cited 11 July 2024]. Available at https://www.aalas.org/about-aalas/position-papers/alleviating-pain-and-d....
-
- American Association for Laboratory Animal Sciences. [Internet]. 2021. Humane care and use of laboratory animals. [Cited 11 July 2024]. Available at https://www.aalas.org/about-aalas/positionpapers/humane-care-and-use.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
