Magnetic Stimulation of Gigantocellular Reticular Nucleus with Iron Oxide Nanoparticles Combined Treadmill Training Enhanced Locomotor Recovery by Reorganizing Cortico-Reticulo-Spinal Circuit
- PMID: 39071504
- PMCID: PMC11283264
- DOI: 10.2147/IJN.S464498
Magnetic Stimulation of Gigantocellular Reticular Nucleus with Iron Oxide Nanoparticles Combined Treadmill Training Enhanced Locomotor Recovery by Reorganizing Cortico-Reticulo-Spinal Circuit
Abstract
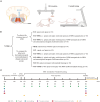
Background: Gigantocellular reticular nucleus (GRNs) executes a vital role in locomotor recovery after spinal cord injury. However, due to its unique anatomical location deep within the brainstem, intervening in GRNs for spinal cord injury research is challenging. To address this problem, this study adopted an extracorporeal magnetic stimulation system to observe the effects of selective magnetic stimulation of GRNs with iron oxide nanoparticles combined treadmill training on locomotor recovery after spinal cord injury, and explored the possible mechanisms.
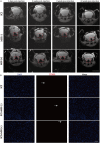
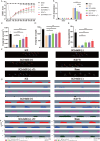
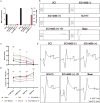
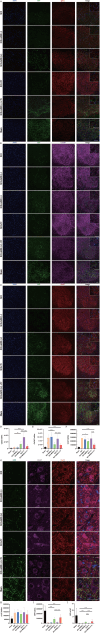
Methods: Superparamagnetic iron oxide (SPIO) nanoparticles were stereotactically injected into bilateral GRNs of mice with moderate T10 spinal cord contusion. Eight-week selective magnetic stimulation produced by extracorporeal magnetic stimulation system (MSS) combined with treadmill training was adopted for the animals from one week after surgery. Locomotor function of mice was evaluated by the Basso Mouse Scale, Grid-walking test and Treadscan analysis. Brain MRI, anterograde virus tracer and immunofluorescence staining were applied to observe the tissue compatibility of SPIO in GRNs, trace GRNs' projections and evaluate neurotransmitters' expression in spinal cord respectively. Motor-evoked potentials and H reflex were collected for assessing the integrity of cortical spinal tract and the excitation of motor neurons in anterior horn.
Results: (1) SPIO persisted in GRNs for a minimum of 24 weeks without inducing apoptosis of GRN cells, and degraded slowly over time. (2) MSS-enabled treadmill training dramatically improved locomotor performances of injured mice, and promoted cortico-reticulo-spinal circuit reorganization. (3) MSS-enabled treadmill training took superimposed roles through both activating GRNs to drive more projections of GRNs across lesion site and rebalancing neurotransmitters' expression in anterior horn of lumbar spinal cord.
Conclusion: These results indicate that selective MSS intervention of GRNs potentially serves as an innovative strategy to promote more spared fibers of GRNs across lesion site and rebalance neurotransmitters' expression after spinal cord injury, paving the way for the structural remodeling of neural systems collaborating with exercise training, thus ultimately contributing to the reconstruction of cortico-reticulo-spinal circuit.
Keywords: gigantocellular reticular nucleus; locomotion; magnetic stimulation; spinal cord injury; superparamagnetic iron oxide nanoparticles; treadmill training.
© 2024 Li et al.
Conflict of interest statement
All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Barthelemy D, Willerslev-Olsen M, Lundell H, Biering-Sorensen F, Nielsen JB. Assessment of transmission in specific descending pathways in relation to gait and balance following spinal cord injury. Prog Brain Res. 2015;218:79–101. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

