Comparative subcutaneous and submuscular implantation of an electroencephalography device for long term electroencephalographic monitoring in dogs
- PMID: 39071780
- PMCID: PMC11272624
- DOI: 10.3389/fvets.2024.1419792
Comparative subcutaneous and submuscular implantation of an electroencephalography device for long term electroencephalographic monitoring in dogs
Abstract
Background: Implantable electroencephalography (EEG) recording devices have been used for ultra-long-term epilepsy monitoring both in clinical and home settings in people. Objective and accurate seizure detection and recording at home could be of great benefit in diagnosis, management and research in canine idiopathic epilepsy (IE). Continuous EEG monitoring would allow accurate detection of seizure patterns, seizure cycles, and seizure frequency. An EEG acquisition system usable in an "out of clinic" setting could improve owner and veterinary compliance for EEG diagnostics and seizure management.
Objectives: Whether a subcutaneous ultra-long term EEG monitoring device designed for humans could be implanted in dogs.
Animals: Cadaver study with 8 medium to large breed dogs.
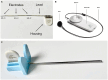
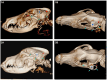
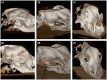
Methods: Comparatively using a subcutaneous and submuscular approach to implant the UNEEG SubQ-Implant in each dog. Positioning was controlled via CT post implantation and cranial measurements were taken.
Results: In four of the eight dogs a submuscular implantation without any complications was possible. Complications were close contact to the optic nerve in the first approaches, before the implantation angle was changed and in the smallest dog contact of the implant with the orbital fat body. Cranial measurements of less than 95 mm length proved to be too small for reliable implantation via this approach. The subcutaneous approach showed severe limitations and the implant was prone to dislocation.
Conclusion: The UNEEQ SubQ-Implant can be implanted in dogs, via submuscular approach. CT imaging and cranial measurements should be taken prior to implantation.
Keywords: EEG; EEG implant; continuous EEG; epilepsy; long-term epilepsy monitoring.
Copyright © 2024 Rogers, Meller, Meyerhoff and Volk.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


Similar articles
-
Ultra-long-term subcutaneous home monitoring of epilepsy-490 days of EEG from nine patients.Epilepsia. 2019 Nov;60(11):2204-2214. doi: 10.1111/epi.16360. Epub 2019 Oct 13. Epilepsia. 2019. PMID: 31608435 Free PMC article.
-
High similarity between EEG from subcutaneous and proximate scalp electrodes in patients with temporal lobe epilepsy.J Neurophysiol. 2018 Sep 1;120(3):1451-1460. doi: 10.1152/jn.00320.2018. Epub 2018 Jul 11. J Neurophysiol. 2018. PMID: 29995605
-
Validation of an EEG seizure detection paradigm optimized for clinical use in a chronically implanted subcutaneous device.J Neurosci Methods. 2021 Jul 1;358:109220. doi: 10.1016/j.jneumeth.2021.109220. Epub 2021 May 7. J Neurosci Methods. 2021. PMID: 33971201
-
A new era in electroencephalographic monitoring? Subscalp devices for ultra-long-term recordings.Epilepsia. 2020 Sep;61(9):1805-1817. doi: 10.1111/epi.16630. Epub 2020 Aug 27. Epilepsia. 2020. PMID: 32852091 Review.
-
Wearable electroencephalography for ultra-long-term seizure monitoring: a systematic review and future prospects.Expert Rev Med Devices. 2021 Dec;18(sup1):57-67. doi: 10.1080/17434440.2021.2012152. Expert Rev Med Devices. 2021. PMID: 34836477
References
LinkOut - more resources
Full Text Sources

