The safety and efficacy profile of eculizumab in myasthenic crisis: a prospective small case series
- PMID: 39072008
- PMCID: PMC11282533
- DOI: 10.1177/17562864241261602
The safety and efficacy profile of eculizumab in myasthenic crisis: a prospective small case series
Abstract
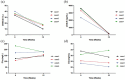
Eculizumab has improved recovery from ventilatory support in myasthenic crisis (MC) cases. However, the safety and efficacy profiles from prospective studies are still lacking. This study aimed to explore eculizumab's safety and efficacy in a prospective case series of patients with refractory MC. We followed a series of anti-acetylcholine receptor (AChR) antibody-positive myasthenia gravis (MG) patients who received eculizumab as an add-on therapy for 12 weeks during MC to facilitate the weaning process and reduced disease activity. Serum anti-AChR antibodies and peripheral immune molecules associated with the complement pathway were evaluated before and after eculizumab administration. Compared to the baseline Myasthenia Gravis Foundation of America (MGFA)-quantitative MG test (QMG) scores (22.25 ± 4.92) and MG-activities of daily living (MG-ADL; 18.25 ± 2.5) scores at crisis, improvements were observed from 4 weeks (14.5 ± 10.47 and 7.5 ± 7.59, respectively) through 12 weeks (7.5 ± 5.74 and 2.25 ± 3.86, respectively) post-treatment. Muscle strength consistently improved across ocular, bulbar, respiratory, and limb/gross domain groups. One patient died of cardiac failure at 16 weeks. Three cases remained in remission at 24 weeks, with a mean QMG score of 2.67 ± 2.89 and ADL score of 0.33 ± 0.58. No significant side effects were reported. Serum CH50 and soluble C5b-9 levels significantly declined, while there were no significant changes in serum anti-AChR antibody levels, C1q, C5a levels, or peripheral lymphocyte proportions. Eculizumab was well tolerated and showed efficacy in this case series. Large prospective cohort studies with extended follow-up periods are needed to further explore the safety and efficacy profile in real-world practice.
Keywords: case report; eculizumab; myasthenic crisis; outcome; rescue therapy.
© The Author(s), 2024.
Figures


References
-
- Punga AR, Maddison P, Heckmann JM, et al.. Epidemiology, diagnostics, and biomarkers of autoimmune neuromuscular junction disorders. Lancet Neurol 2022; 21: 176–188. - PubMed
-
- Ahmed S, Kirmani JF, Janjua N, et al. An update on myasthenic crisis. Curr Treat Options Neurol 2005; 7: 129–141. - PubMed
-
- Wang Y, Huan X, Jiao K, et al. Plasma exchange versus intravenous immunoglobulin in AChR subtype myasthenic crisis: a prospective cohort study. Clin Immunol 2022; 241: 109058. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

