This is a preprint.
Spatial and temporal transmission dynamics of respiratory syncytial virus in New Zealand before and after the COVID-19 pandemic
- PMID: 39072023
- PMCID: PMC11275701
- DOI: 10.1101/2024.07.15.24310412
Spatial and temporal transmission dynamics of respiratory syncytial virus in New Zealand before and after the COVID-19 pandemic
Update in
-
Spatial and temporal transmission dynamics of respiratory syncytial virus in New Zealand before and after the COVID-19 pandemic.Nat Commun. 2024 Nov 11;15(1):9758. doi: 10.1038/s41467-024-53998-5. Nat Commun. 2024. PMID: 39528493 Free PMC article.
Abstract
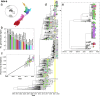
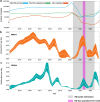
Human respiratory syncytial virus (RSV) is a major cause of acute respiratory infection. In 2020, RSV was effectively eliminated from the community in New Zealand due to non-pharmaceutical interventions (NPI) used to control the spread of COVID-19. However, in April 2021, following a brief quarantine-free travel agreement with Australia, there was a large-scale nationwide outbreak of RSV that led to reported cases more than five times higher, and hospitalisations more than three times higher, than the typical seasonal pattern. In this study, we generated 1,471 viral genomes of both RSV-A and RSV-B sampled between 2015 and 2022 from across New Zealand. Using a phylodynamics approach, we used these data to better understand RSV transmission patterns in New Zealand prior to 2020, and how RSV became re-established in the community following the relaxation of COVID-19 restrictions. We found that in 2021, there was a large epidemic of RSV in New Zealand that affected a broader age group range compared to the usual pattern of RSV infections. This epidemic was due to an increase in RSV importations, leading to several large genomic clusters of both RSV-A ON1 and RSV-B BA9 genotypes in New Zealand. However, while a number of importations were detected, there was also a major reduction in RSV genetic diversity compared to pre-pandemic seasonal outbreaks. These genomic clusters were temporally associated with the increase of migration in 2021 due to quarantine-free travel from Australia at the time. The closest genetic relatives to the New Zealand RSV genomes, when sampled, were viral genomes sampled in Australia during a large, off-season summer outbreak several months prior, rather than cryptic lineages that were sustained but not detected in New Zealand. These data reveal the impact of NPI used during the COVID-19 pandemic on other respiratory infections and highlight the important insights that can be gained from viral genomes.
Keywords: New Zealand; RSV; Respiratory syncytial virus; disease outbreak; genomic epidemiology; phylodynamics; virus evolution.
Figures


References
-
- Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. New England Journal of Medicine. 2005; 352(17):1749–59. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
