Silica-Reinforced AMP-Calcium Alginate Beads for Efficient and Selective Removal of Cesium from a Strong Acidic Medium
- PMID: 39072054
- PMCID: PMC11270694
- DOI: 10.1021/acsomega.4c03806
Silica-Reinforced AMP-Calcium Alginate Beads for Efficient and Selective Removal of Cesium from a Strong Acidic Medium
Abstract
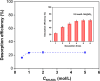
Due to the significant selectivity for Cs+, ammonium molybdophosphate (AMP) possesses potential to uptake radiocesium from high-level liquid waste (HLLW), whereas its micro-crystalline structure and fine powder morphology limit its industrial application. Although the granulation method with alginate is prospective for the preparation of an AMP exchanger, the mechanical strength of obtained beads may be insufficient for application. In this context, we prepared silica-reinforced AMP-calcium alginate (ACS) beads and evaluated their performance for Cs+ removal from strong acidic solutions. It was found that the addition of silica in the fabrication significantly improved the mechanical strength of the beads in comparison to those without silica. Notably, the beads with an AMP/silica mass ratio of 1.0 exhibited an exceptional mechanical strength, surpassing that of ACS beads composed of other components. The batch experiment results indicated that the Cs+ adsorption follows a non-linear pseudo-second-order rate equation. The distribution coefficient of Cs+ was high even in extreme acidic conditions (∼1.6 × 102 mL/g in 8.0 mol/L HNO3 solution). The Cs+ adsorption can be well fitted with the Langmuir model, and the estimated maximum exchange capacity in 3.0 mol/L HNO3 could reach 23.9 mg/g. More importantly, ACS beads showed excellent selectivity toward Cs+ uptake over eight co-existing metal ions in simulated HLLW, with separation factor values all above 145. The column experiment exhibited that the beads can serve as the stationary phase in columns to effectively remove Cs+. The findings of this study are significant as they provide insights into the development of efficient materials for radiocesium removal from high-level liquid waste. The results demonstrate the potential of silica-reinforced ACS beads for Cs+ adsorption, with promising applications in industrial settings.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures










References
-
- Yoshikawa H.; Sunada S.; Hirakawa H.; Fujimori A.; Elmegerhi S.; Leary D.; Kato T. A. Radiobiological characterization of canine malignant melanoma cell lines with different types of ionizing radiation and efficacy evaluation with cytotoxic agents. Int. J. Mol. Sci. 2019, 20, 841.10.3390/ijms20040841. - DOI - PMC - PubMed
-
- Artun O. A study of nuclear structure for 244Cm, 241Am, 238Pu, 210Po, 147Pm, 137Cs, 90Sr and 63Ni nuclei used in nuclear battery. Mod. Phys. Lett. 2017, 32, 1750117.10.1142/S0217732317501176. - DOI
-
- Tosri C.; Chusreeaeom K.; Limtiyayotin M.; Sukin N.; Jompuk P. Comparative effect of high energy electron beam and 137Cs gamma ray on survival, growth and chlorophyll content in curcuma hybrid ‘laddawan’ and determine proper dose for mutations breeding. Emir. J. Food. Agr. 2019, 31, 321–327. 10.9755/ejfa.2019.v31.i5.1942. - DOI
-
- Wang J. L.; Zhang S. Removal of cesium ions from aqueous solutions using various separation technologies. Rev. Environ. Sci. Bio/Technol. 2019, 18, 231–269. 10.1007/s11157-019-09499-9. - DOI
LinkOut - more resources
Full Text Sources
