Managing Oxidative Stress Using Vitamin C to Improve Biocompatibility of Polycaprolactone for Bone Regeneration In Vitro
- PMID: 39072128
- PMCID: PMC11270701
- DOI: 10.1021/acsomega.4c02858
Managing Oxidative Stress Using Vitamin C to Improve Biocompatibility of Polycaprolactone for Bone Regeneration In Vitro
Abstract
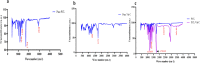
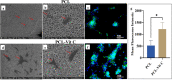
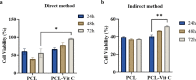
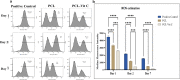
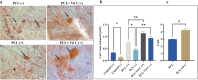
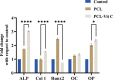
Increased oxidative stress in bone cells is known to negatively alter favorable bone regeneration. This study aimed to develop a porous polycaprolactone (PCL) membrane incorporated with 25 wt % Vitamin C (PCL-Vit C) and compared it to the PCL membrane to control oxidative stress and enhance biomineralization in vitro. Both membranes were characterized using SEM-EDS, FTIR spectroscopy, and surface hydrophilicity. Vitamin C release was quantified colorimetrically. Assessments of the viability and attachment of human fetal osteoblast (hFOB 1.19) cells were carried out using XTT assay, SEM, and confocal microscopy, respectively. ROS generation and wound healing percentage were measured using flow cytometry and ImageJ software, respectively. Mineralization study using Alizarin Red in the presence or absence of osteogenic media was carried out to measure the calcium content. Alkaline phosphatase assay and gene expression of osteogenic markers (alkaline phosphatase (ALP), collagen Type I (Col1), runt-related transcription factor 2 (RUNX2), osteocalcin (OCN), and osteopontin (OPN)) were analyzed by real-time PCR. SEM images revealed smooth, fine, bead-free fibers in both membranes. The FTIR spectrum of pure vitamin C was replaced with peaks at 3436.05 and 2322.83 cm-1 in the PCL-Vit C membrane. Vitamin C release was detected at 15 min and 1 h. The PCL-Vit C membrane was hydrophilic, generated lower ROS, and showed significantly higher viability than the PCL membrane. Although both PCL and PCL-Vit C membranes showed similar cellular and cytoskeletal morphology, more cell clusters were evident in the PCL-Vit C membrane. Lower ROS level in the PCL-Vit C membrane displayed improved cell functionality as evidenced by enhanced cellular differentiation with more intense alizarin staining and higher calcium content, supported by upregulation of osteogenic markers ALP, Col1, and OPN even in the absence of osteogenic supplements. The presence of Vitamin C in the PCL-Vit C membrane may have mitigated oxidative stress in hFOB 1.19 cells, resulting in enhanced biomineralization facilitating bone regeneration.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Chang H.-H.; Guo M.-K.; Kasten F. H.; Chang M.-C.; Huang G.-F.; Wang Y.-L.; Wang R.-S.; Jeng J.-H. Stimulation of glutathione depletion, ROS production and cell cycle arrest of dental pulp cells and gingival epithelial cells by HEMA. Biomaterials 2005, 26 (7), 745–753. 10.1016/j.biomaterials.2004.03.021. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
