Aldehyde dehydrogenase 2 family member repression promotes colorectal cancer progression by JNK/p38 MAPK pathways-mediated apoptosis and DNA damage
- PMID: 39072174
- PMCID: PMC11271775
- DOI: 10.4251/wjgo.v16.i7.3230
Aldehyde dehydrogenase 2 family member repression promotes colorectal cancer progression by JNK/p38 MAPK pathways-mediated apoptosis and DNA damage
Abstract
Background: Aldehyde (ALDH2) dysfunction has been verified to contribute to human cancers.
Aim: To investigate the molecular mechanism and biological function of ALDH2 in colorectal cancer (CRC) progression.
Methods: Human CRC cells with high expression of ALDH2 were screened. After shRNA ALDH2 (sh-ALDH2) transfection, phenotypes [proliferation, apoptosis, acetaldehyde (ACE) accumulation, DNA damage] of CRC cells were verified using cell counting kit-8, flow cytometry, ACE assay, and comet assays. Western blotting was used for evaluation of the apoptosis proteins (Bax and Bcl-2) and JNK/p38 MAPK pathway-associated proteins. We subjected CVT-10216 (a selective ALDH2 inhibitor) to nude mice for establishment of SK-CO-1 mouse xenograft model and observed the occurrence of CRC.
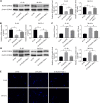
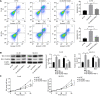
Results: The inhibition of ALDH2 could promote the malignant structures of CRC cells, including apoptosis, ACE level, and DNA damage, and cell proliferation was decreased in the sh-ALDH2 group, whereas ALDH2 agonist Alda-1 reversed features. ALDH2 repression can cause ACE accumulation, whereas ACE enhanced CRC cell features related to increased DNA damage. Additionally, ALDH2 repression led to JNK/P38 MAPK activation, and apoptosis, ACE accumulation, and DNA damage were inhibited after p38 MAPK inhibitor SB203580 and JNK inhibitor SP600125 addition. ACE accumulation and raised DNA damage were recognized in CVT-10216 treated-mouse tumor tissues in vivo.
Conclusion: The repression of ALDH2 led to ACE accumulation, inducing cell apoptosis and DNA damage by the JNK/p38 MAPK signaling pathway activation in CRC.
Keywords: Acetaldehyde; Aldehyde dehydrogenase 2 family member; Apoptosis; Colorectal cancer; DNA damage; JNK/p38 MAPK.
©The Author(s) 2024. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All authors have nothing to disclose.
Figures




References
-
- Siegel RL, Jakubowski CD, Fedewa SA, Davis A, Azad NS. Colorectal Cancer in the Young: Epidemiology, Prevention, Management. Am Soc Clin Oncol Educ Book. 2020;40:1–14. - PubMed
-
- Morgan E, Arnold M, Gini A, Lorenzoni V, Cabasag CJ, Laversanne M, Vignat J, Ferlay J, Murphy N, Bray F. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. 2023;72:338–344. - PubMed
-
- Aran V, Victorino AP, Thuler LC, Ferreira CG. Colorectal Cancer: Epidemiology, Disease Mechanisms and Interventions to Reduce Onset and Mortality. Clin Colorectal Cancer. 2016;15:195–203. - PubMed
-
- Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467–1480. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

