Flow cytometry-based diagnostic approach for inborn errors of immunity: experience from Algeria
- PMID: 39072316
- PMCID: PMC11273131
- DOI: 10.3389/fimmu.2024.1402038
Flow cytometry-based diagnostic approach for inborn errors of immunity: experience from Algeria
Abstract
Purpose: In this study, we retrospectively reviewed the use of flow cytometry (FCM) in the diagnosis of inborn errors of immunity (IEIs) at a single center in Algeria. Sharing insights into our practical experience, we present FCM based diagnostic approaches adapted to different clinical scenarios.
Methods: Between May 2017 and February 2024, pediatric and adult patients presenting with clinical features suggestive of immunodeficiency were subjected to FCM evaluation, including lymphocyte subset analysis, detection of specific surface or intracellular proteins, and functional analysis of immune cells.
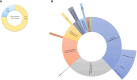
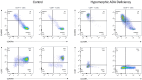
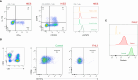
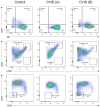
Results: Over a nearly seven-year period, our laboratory diagnosed a total of 670 patients (372 (55.5%) males and 298 (44.5%) females), distributed into 70 different IEIs belonging to 9 different categories of the International Union of Immunological Societies classification. FCM was used to diagnose and categorize IEI in 514 patients (76.7%). It provided direct diagnostic insights for IEIs such as severe combined immunodeficiency, Omenn syndrome, MHC class II deficiency, familial hemophagocytic lymphohistiocytosis, and CD55 deficiency. For certain IEIs, including hyper-IgE syndrome, STAT1-gain of function, autoimmune lymphoproliferative syndrome, and activated PI3K delta syndrome, FCM offered suggestive evidence, necessitating subsequent genetic testing for confirmation. Protein expression and functional assays played a crucial role in establishing definitive diagnoses for various disorders. To setup such diagnostic assays at high and reproducible quality, high level of expertise is required; in house reference values need to be determined and the parallel testing of healthy controls is highly recommended.
Conclusion: Flow cytometry has emerged as a highly valuable and cost-effective tool for diagnosing and studying most IEIs, particularly in low-income countries where access to genetic testing can be limited. FCM analysis could provide direct diagnostic insights for most common IEIs, offer clues to the underlying genetic defects, and/or aid in narrowing the list of putative genes to be analyzed.
Keywords: complement deficiencies; flow cytometry; flow cytometry-based diagnostic approach; functional assays; lymphocyte phenotyping; protein expression assays.
Copyright © 2024 Tahiat, Belbouab, Yagoubi, Hakem, Fernini, Keddari, Belhadj, Touri, Aggoune, Stoddard, Niemela, Zerifi, Melzi, Aboura, Saad-Djaballah, Ferhani, Ketfi, Messaoudi, Bencharif Madani, Benhacine, Dehimi, Okka, Amroune, Fellahi, Bendahmane, Khoulani, Oukil, Soufane, Bourelaf, Boubidi, Boukhenfouf, Amine Ifri, Khelafi, Boudiaf, Khelifi Touhami, Meçabih, Boucelma, Zelaci, Gacem, Ladj, Mekki, Bensaadi, Benhalima, Zeroual, Bioud, Benameur, Bouhdjila, Bouzerar, Ibsaine, Maouche, Kedji, Smati, Boukari, Lambert, Rosenzweig, Notarangelo and Djenouhat.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
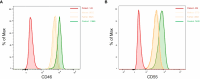
References
-
- Tangye SG, Al-Herz W, Bousfiha A, Cunningham-Rundles C, Franco JL, Holland SM, et al. Human inborn errors of immunity: 2022 update on the classification from the international union of immunological societies expert committee. J Clin Immunol. (2022) 42:1473–507. doi: 10.1007/s10875-022-01289 - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

