Stevens-Johnson syndrome and toxic epidermal necrolysis associated with immune checkpoint inhibitors: a systematic review
- PMID: 39072330
- PMCID: PMC11272453
- DOI: 10.3389/fimmu.2024.1414136
Stevens-Johnson syndrome and toxic epidermal necrolysis associated with immune checkpoint inhibitors: a systematic review
Abstract
Introduction: Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are rare yet life-threatening adverse events associated with immune checkpoint inhibitors (ICIs). This systematic review synthesizes the current literature to elucidate the clinical characteristics and outcomes of patients with ICI-related SJS/TEN.
Methods: We conducted a thorough search across databases including Embase, Web of Science, Cochrane, MEDLINE, Scopus, and PubMed. Selection criteria focused on reports of SJS/TEN among cancer patients treated with ICIs, analyzing clinical manifestations, therapeutic interventions, and outcomes.
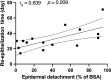
Results: Our analysis included 47 articles involving 50 patients with ICI-related SJS/TEN. The cohort had a mean age of 63 years, with a slight male predominance (54%). Most patients had melanoma or non-small cell lung cancer. SJS/TEN typically occurred early, with a median onset of 23 days post-ICI initiation. Treatment primarily involved systemic corticosteroids and intravenous immunoglobulins. The overall mortality rate was 20%, higher for TEN at 32%, with infections and tumor progression as leading causes. Median time from onset to death was 28 days. Survivors experienced a median re-epithelization time of 30 days, positively correlated with the extent of epidermal detachment (rs = 0.639, p = 0.009). Deceased patients exhibited a significantly higher proportion of TEN (90% vs. 48%, p = 0.029) and a larger epidermal detachment area (90% vs. 30% of the body surface area [BSA], p = 0.005) compared to survivors. The combination therapy group showed a higher proportion of TEN compared to corticosteroid monotherapy or non-corticosteroid therapy groups (72% vs. 29% and 50%, p = 0.01), with no significant differences in mortality or re-epithelization time. Dual ICI therapy resulted in a higher TEN rate than single therapy (100% vs. 50%, p = 0.028). Among single ICI therapies, the sintilimab-treated group trended towards a higher TEN rate (75% vs. 40-50%, p = 0.417), a larger detachment area (90% vs. 30-48% of BSA, p = 0.172), and a longer re-epithelization time (44 vs. 14-28 days, p = 0.036) compared to other ICI groups, while mortality rates remained similar.
Conclusion: ICI-related SJS/TEN substantially impacts patient outcomes. Prospective clinical trials are critically needed to further clarify the pathogenesis and optimize therapeutic regimens.
Keywords: Stevens-Johnson syndrome; immune checkpoint inhibitor; immune-related adverse events; irAE; severe cutaneous adverse reaction; toxic epidermal necrolysis.
Copyright © 2024 Zhou, Wang, Li, Zhang and He.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

