VERITAC-2: a Phase III study of vepdegestrant, a PROTAC ER degrader, versus fulvestrant in ER+/HER2- advanced breast cancer
- PMID: 39072356
- PMCID: PMC11524203
- DOI: 10.1080/14796694.2024.2377530
VERITAC-2: a Phase III study of vepdegestrant, a PROTAC ER degrader, versus fulvestrant in ER+/HER2- advanced breast cancer
Abstract
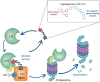
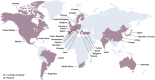
Vepdegestrant (ARV-471) is an oral PROTAC ER degrader that binds an E3 ubiquitin ligase and ER to directly trigger ubiquitination of ER and its subsequent proteasomal degradation. In a first-in-human Phase I/II study, vepdegestrant monotherapy was well tolerated with clinical activity in pretreated patients with ER+/HER2- advanced breast cancer. The global, randomized Phase III VERITAC-2 study compares efficacy and safety of vepdegestrant versus fulvestrant in adults with ER+/HER2- advanced breast cancer after treatment with a CDK4/6 inhibitor plus endocrine therapy. Progression-free survival by blinded independent central review (primary end point) will be assessed in the intention-to-treat population and ESR1 mutation-positive subpopulation. Secondary end points include overall survival, tumor response, safety, pharmacokinetics, patient-reported outcomes, and circulating tumor DNA biomarkers.Clinical trial registration: NCT05654623 (ClinicalTrials.gov).
Keywords: ARV-471; ER+; HER2-; PROTAC; breast cancer; fulvestrant; targeted protein degradation; vepdegestrant.
Plain language summary
VERITAC-2 is a clinical trial comparing vepdegestrant, a new drug that degrades estrogen receptors, to an existing treatment called fulvestrant in patients with ER+/HER2- advanced breast cancer: Estrogen receptor-positive (ER+)/human epidermal growth factor receptor 2-negative (HER2-) breast cancer grows in response to estrogen, a hormone in the body, and has low levels or no HER2 protein. People living with ER+/HER2- advanced breast cancer that has grown, spread to another part of the body, or cannot be removed by surgery are often treated with cyclin-dependent kinase (CDK) 4/6 inhibitors and endocrine therapies, but their cancer may get worse on these treatments and new treatments are needed. Fulvestrant, an endocrine therapy that attaches to estrogen receptors, lowers estrogen's effect on tumors and can slow or stop cancer growth. Vepdegestrant, a new medicine being tested for ER+ breast cancer, is a PROteolysis TArgeting Chimera (PROTAC) protein degrader that attaches to estrogen receptors and causes them to be tagged for removal by the cell's natural protein disposal system. By removing estrogen receptors, vepdegestrant may cause tumors to stop growing or shrink.This paper describes the Phase III VERITAC-2 clinical study comparing vepdegestrant versus fulvestrant in people living with ER+/HER2- advanced breast cancer previously treated with a CDK4/6 inhibitor and endocrine therapy.Patients will be randomly assigned to receive vepdegestrant (a pill taken once daily by mouth) or fulvestrant (a shot given into the muscle). The purpose of the study is to find out how long people live without their cancer getting worse with vepdegestrant or fulvestrant. VERITAC-2 will also look at how long people live during the study, side effects people may experience, and the overall well-being of people throughout the study.
Conflict of interest statement
EP Hamilton has served in a consulting/advisory role for Arcus, AstraZeneca, Daiichi Sankyo, Ellipses Pharma, Genentech/Roche, Greenwich LifeSciences, iTeos, Janssen, Lilly, Loxo Oncology, Mersana, Novartis, Olema Pharmaceuticals, Orum Therapeutics, Pfizer, Inc., Relay Therapeutics, SeaGen, Stemline Therapeutics, Theratechnologies, Tubulis, and Verascity Science; received institutional research support from AbbVie, Accutar Biotechnology, Acerta Pharma, ADC Therapeutics, Akesobio Australia, Amgen, Aravive, Arqule, Artios, Arvinas, Inc., AstraZeneca, Atlas, BeiGene, Black Diamond, Bliss BioPharmaceuticals, Boehringer Ingelheim, Cascadian Therapeutics, Clovis, Compugen, Context Therapeutics, Cullinan-Florentine, Curis, CytomX, Daiichi Sankyo, Dana Farber Cancer Hospital, Dantari, Deciphera, Duality Biologics, eFFECTOR Therapeutics, Ellipses, Elucida Oncology, EMD Serono, FujiFilm, G1 Therapeutics, Genentech/Roche, H3 Biomedicine, Harpoon, Hutchinson MediPharma, Immunogen, Immunomedics, Incyte, Infinity Pharmaceuticals, InventisBio, Jacobio, K-Group Beta, Karyopharm, Kind Pharmaceuticals, Leap Therapeutics, Lilly, Loxo Oncology, Lycera, MabSpace Biosciences, Macrogenics, MedImmune, Mersana, Merus, Millenium, Molecular Templates, Novartis, Nucana, Olema, Oncomed, Onconova Therapeutics, Oncothyreon, ORIC Pharmaceuticals, Orinove, Orum Therapeutics, Pfizer, Inc., PharmaMar, Pieris Pharmaceuticals, Pionyr Immunotherapeutics, Plexxicon, Prelude Therapeutics, Profound Bio, Radius Health, Regeneron, Relay Therapeutics, Repertoire Immune Medicine, Rgenix, SeaGen, Sermonix Pharmaceuticals, Shattuck Labs, StemCentRx, Sutro, Syndax, Syros, Taiho, TapImmune, Tesaro, Tolmar, Torque Therapeutics, Treadwell Therapeutics, Verastem, Zenith Epigenetics, and Zymeworks. C Ma has served in a consulting role for AstraZeneca, Daiichi Sankyo, Genzyme, Gilead Sciences, Lilly, Novartis, Olaris, Pfizer, Inc., Regor, Stemline, Tempus, and TerSera; and has received research funding from Pfizer, Inc. and Puma Biotechnology. M De Laurentiis has received personal fees from AstraZeneca, Celgene, Daiichi Sankyo, Eisai, Eli Lilly, Exact Science, Gilead, Ipsen, Menarini-Stemline, MSD, Novartis, Pfizer, Inc., Pierre Fabre, Roche, Seagen, Takeda, and Veracyte, outside the submitted work. He has stock/ownership interests in Arvinas, Inc. H Iwata has served as invited speaker/advisory board member for AstraZeneca, Chugai, Daiichi Sankyo, Lilly, Pfizer, Sanofi, and Taiho; served as a steering committee member for Amgen, AstraZeneca, Chugai, Daiichi Sankyo, Kyowa Hakko Kirin, MSD, Novartis, Pfizer, Inc., and Sanofi; and served as a local principal investigator for Bayer, Boehringer, Lilly, and Nihon Kayaku. SA Hurvitz has served as an invited speaker for Axis Medical, Cancer Expert Now, Clinical Care Options, ICHE, MJH Associates, Peer Education, PER, PrecisCA, Primo, Projects in Knowledge, Prova Education, Research to Practice, Ultimate Medical Academy, Vaniam, and WebMD; has ownership interest in Ideal Implant and stocks and shares in ROM Tech; she receives royalties from Elsevier, McGraw, Sage, Springer, Wiley, and Wolters Kluwer; is a local principal investigator for Ambrx, Arvinas, Inc., AstraZeneca, Dantari, Dignitana, Eli Lilly, G1 Therapeutics, Genentech/Roche, Gilead, Greenwich Life Sciences, GSK, Immunomedics, Loxo Oncology, Macrogenics, Novartis, OBI Pharma, Orinove, Orum, Pfizer, Inc., Phoenix Molecular Designs, Pieris, Puma Biotechnology, Radius, Sanofi, Seattle Genetics, and Zymeworks; serves as coordinating principal investigator for Celcuity and Daiichi Sankyo; and has received research funding from Ambrx and Samumed. SA Hurvitz serves as a steering committee member for Greenwich Life Sciences and Orum; serves as principal investigator for Daiichi Sankyo, Genentech, and Seattle Genetics; and advisor for 4DPharma, Ambrx, Amgen, Artios, Arvinas, Inc., Daiichi Sankyo, Dantari, Immunomedics/Gilead, Lilly, Macrogenics, Novartis, Pieris, Pyxis, and Roche. SA Wander has served in a consulting/advisory board role for AstraZeneca, Biovica, Eli Lilly, Foundation Medicine, Genentech, Hologic, Novartis, Pfizer, Inc., Puma Biotechnology, and Veracyte; has done educational speaking for Eli Lilly, Guardant Health, and 2ndMD; and has received institutional research support from Eli Lilly, Genentech, Nuvation Bio, Pfizer, Inc., Regor Therapeutics Group, and Sermonix. M Danso has served in a consulting/advisory role for Immunomedics, Novartis, Pfizer, Inc., and Seattle Genetics; and has received honoraria from Amgen. DR Lu is employed by and has stock/ownership interests in Pfizer, Inc. J Perkins Smith is employed by and has stock/other ownership interests in Pfizer, Inc. Y Liu is employed by and has stock/ownership interests in Pfizer, Inc. L Tran is employed by and has stock/ownership interests in Pfizer, Inc. S Anderson was employed by Arvinas, Inc. at the time of manuscript development and had/has stock/ownership interests in Arvinas, Inc. M Campone has served in a consulting/advisory role for AstraZeneca, Daiichi Sankyo, Diaccurate, Gilead, Lilly, Menarini, Novartis, PET Therapy, Pfizer, Inc., Sanofi, and Seagen; has served as invited speaker for Lilly and Novartis; and received travel reimbursement fees from AstraZeneca, Lilly, Novartis, Pfizer, Inc., and Roche. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures


References
-
- Lloyd MR, Wander SA, Hamilton E, et al. . Next-generation selective estrogen receptor degraders and other novel endocrine therapies for management of metastatic hormone receptor-positive breast cancer: current and emerging role. Ther Adv Med Oncol. 2022;14:1–25. doi:10.1177/17588359221113694 - DOI - PMC - PubMed
-
- Wander SA, Cohen O, Gong X, et al. . The genomic landscape of intrinsic and acquired resistance to cyclin-dependent kinase 4/6 inhibitors in patients with hormone receptor-positive metastatic breast cancer. Cancer Discov. 2020;10(8):1174–1193. doi:10.1158/2159-8290.CD-19-1390 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
