Renal IL-23-Dependent Type 3 Innate Lymphoid Cells Link Crystal-induced Intrarenal Inflammasome Activation with Kidney Fibrosis
- PMID: 39072698
- PMCID: PMC11372247
- DOI: 10.4049/jimmunol.2400041
Renal IL-23-Dependent Type 3 Innate Lymphoid Cells Link Crystal-induced Intrarenal Inflammasome Activation with Kidney Fibrosis
Abstract
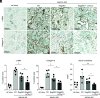
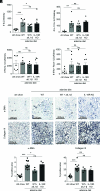
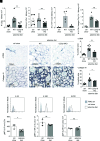
Chronic inflammasome activation in mononuclear phagocytes (MNPs) promotes fibrosis in various tissues, including the kidney. The cellular and molecular links between the inflammasome and fibrosis are unclear. To address this question, we fed mice lacking various immunological mediators an adenine-enriched diet, which causes crystal precipitation in renal tubules, crystal-induced inflammasome activation, and renal fibrosis. We found that kidney fibrosis depended on an intrarenal inflammasome-dependent type 3 immune response driven by its signature transcription factor Rorc (retinoic acid receptor-related orphan receptor C gene), which was partially carried out by type 3 innate lymphoid cells (ILC3s). The role of ILCs in the kidney is less well known than in other organs, especially that of ILC3. In this article, we describe that depletion of ILCs or genetic deficiency for Rorc attenuated kidney inflammation and fibrosis. Among the inflammasome-derived cytokines, only IL-1β expanded ILC3 and promoted fibrosis, whereas IL-18 caused differentiation of NKp46+ ILC3. Deficiency of the type 3 maintenance cytokine, IL-23, was more protective than IL-1β inhibition, which may be explained by the downregulation of the IL-1R, but not of the IL-23R, by ILC3 early in the disease, allowing persistent sensing of IL-23. Mechanistically, ILC3s colocalized with renal MNPs in vivo as shown by multiepitope-ligand cartography. Cell culture experiments indicated that renal ILC3s caused renal MNPs to increase TGF-β production that stimulated fibroblasts to produce collagen. We conclude that ILC3s link inflammasome activation with kidney inflammation and fibrosis and are regulated by IL-1β and IL-23.
Copyright © 2024 by The American Association of Immunologists, Inc.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures



References
MeSH terms
Substances
Grants and funding
- 36842431/Deutsche Forschungsgemeinschaft (DFG)
- 273942223/Deutsche Forschungsgemeinschaft (DFG)
- 264599542/Deutsche Forschungsgemeinschaft (DFG)
- 272482170/Deutsche Forschungsgemeinschaft (DFG)
- 432325352/Deutsche Forschungsgemeinschaft (DFG)
- 386793560/Deutsche Forschungsgemeinschaft (DFG)
- 466687329/Deutsche Forschungsgemeinschaft (DFG)
- 322900939/Deutsche Forschungsgemeinschaft (DFG)
- 454024652/Deutsche Forschungsgemeinschaft (DFG)
- 432698239/Deutsche Forschungsgemeinschaft (DFG)
- 445703531/Deutsche Forschungsgemeinschaft (DFG)
- 390873048/Deutsche Forschungsgemeinschaft (DFG)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

