Drosophila HCN mediates gustatory homeostasis by preserving sensillar transepithelial potential in sweet environments
- PMID: 39073076
- PMCID: PMC11286260
- DOI: 10.7554/eLife.96602
Drosophila HCN mediates gustatory homeostasis by preserving sensillar transepithelial potential in sweet environments
Abstract
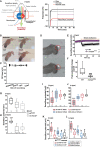
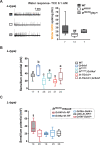
Establishing transepithelial ion disparities is crucial for sensory functions in animals. In insect sensory organs called sensilla, a transepithelial potential, known as the sensillum potential (SP), arises through active ion transport across accessory cells, sensitizing receptor neurons such as mechanoreceptors and chemoreceptors. Because multiple receptor neurons are often co-housed in a sensillum and share SP, niche-prevalent overstimulation of single sensory neurons can compromise neighboring receptors by depleting SP. However, how such potential depletion is prevented to maintain sensory homeostasis remains unknown. Here, we find that the Ih-encoded hyperpolarization-activated cyclic nucleotide-gated (HCN) channel bolsters the activity of bitter-sensing gustatory receptor neurons (bGRNs), albeit acting in sweet-sensing GRNs (sGRNs). For this task, HCN maintains SP despite prolonged sGRN stimulation induced by the diet mimicking their sweet feeding niche, such as overripe fruit. We present evidence that Ih-dependent demarcation of sGRN excitability is implemented to throttle SP consumption, which may have facilitated adaptation to a sweetness-dominated environment. Thus, HCN expressed in sGRNs serves as a key component of a simple yet versatile peripheral coding that regulates bitterness for optimal food intake in two contrasting ways: sweet-resilient preservation of bitter aversion and the previously reported sweet-dependent suppression of bitter taste.
Keywords: D. melanogaster; HCN; feeding behavior; hyperpolarization-activated cyclic nucleotide-gated; neuronal microenvironment; neuroscience; sensillum potential; sensory homeostasis; sensory processing at the periphery.
© 2024, Lee et al.
Conflict of interest statement
ML, SP, KJ, JK, KL, KK No competing interests declared
Figures








Update of
- doi: 10.1101/2024.02.06.579099
- doi: 10.7554/eLife.96602.1
- doi: 10.7554/eLife.96602.2
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

