Scenarios for the long-term efficacy of amyloid-targeting therapies in the context of the natural history of Alzheimer's disease
- PMID: 39073291
- PMCID: PMC11497713
- DOI: 10.1002/alz.14134
Scenarios for the long-term efficacy of amyloid-targeting therapies in the context of the natural history of Alzheimer's disease
Abstract
Introduction: Recent clinical trials of amyloid beta (Aβ)-targeting therapies in Alzheimer's disease (AD) have demonstrated a clinical benefit over 18 months, but their long-term impact on disease trajectory is not yet understood. We propose a framework for evaluating realistic long-term scenarios.
Methods: Results from recent phase 3 trials of Aβ-targeting antibodies were integrated with an estimate of the long-term patient-level natural history trajectory of the Clinical Dementia Rating-Sum of Boxes (CDR-SB) score to explore realistic long-term efficacy scenarios.
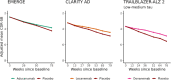
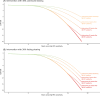
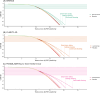
Results: Three distinct long-term efficacy scenarios were examined, ranging from conservative to optimistic. These extrapolations of positive phase 3 trials suggested treatments delayed onset of severe dementia by 0.3 to 0.6 years (conservative), 1.1 to 1.9 years (intermediate), and 2.0 to 4.2 years (optimistic).
Discussion: Our study provides a common language for long-term impact of disease-modifying treatments. Our work calls for studies with longer follow-up and results from early intervention trials to provide a comprehensive assessment of these therapies' true long-term impact.
Highlights: We present long-term scenarios of the efficacy of AD therapies. In this framework, scenarios are defined relative to the natural history of AD. Long-term projections with different levels of optimism can be compared. It provides a common language for expressing beliefs about long-term efficacy.
Keywords: amyloid‐targeting therapies; clinical trials; disease modeling; disease modification; long‐term efficacy; time saving.
© 2024 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
LLR is an employee and shareholder of Eli Lilly and Company. JC has provided consultation to Acadia, Actinogen, Acumen, AlphaCognition, Aprinoia, AriBio, Artery, Biogen, BioVie, Cassava, Cerecin, Diadem, EIP Pharma, Eisai, GemVax, Genentech, GAP Innovations, Janssen, Jocasta, Karuna, Lilly, Lundbeck, LSP, Merck, NervGen, Novo Nordisk, Oligomerix, Optoceutics, Ono, Otsuka, PRODEO, Prothena, ReMYND, Roche, Sage Therapeutics, Signant Health, Simcere, Suven, SynapseBio, TrueBinding, Vaxxinity, and Wren pharmaceutical, assessment, and investment companies. AM reports no conflicts of interest. NV has received research support from Fondation Bettencourt‐Schueller, Fondation Servier, Union Nationale pour les Intárêts de la Mádecine (UNIM), Fondation Claude Pompidou, Fondation Alzheimer and Fondation pour la Recherche sur l'Alzheimer; travel grants from the Movement Disorders Society, Merz‐Pharma, UCB Pharma, and GE Healthcare SAS; is an unpaid local principal investigator or subinvestigator in NCT04241068 and NCT05310071 (aducanumab, Biogen), NCT05399888 (BIIB080, Biogen), NCT03352557 (gosuranemab, Biogen), NCT05463731 (remternetug, Eli Lilly), NCT04592341 (gantenerumab, Roche), NCT03887455 (lecanemab, Eisai), NCT03828747 and NCT03289143 (semorinemab, Roche), NCT04619420 (JNJ‐63733657, Janssen—Johnson & Johnson), NCT04374136 (AL001, Alector), NCT04592874 (AL002, Alector), NCT04867616 (bepranemab, UCB Pharma), NCT04777396 and NCT04777409 (semaglutide, Novo Nordisk), NCT05469360 (NIO752, Novartis), is an unpaid national coordinator for NCT05564169 (masitinib, ABScience), NCT (AD04, ADvantage Therapeutics GmbH); has given unpaid lectures at symposia organized by Eisai and the Servier Foundation; and has been an unpaid expert for Janssen and Johnson & Johnson, all outside of this work. MS has served on advisory boards for and received funding from Roche Diagnostics and Novo Nordisk (outside scope of submitted work). Author disclosures are available in the supporting information.
Figures
References
-
- Villain N, Planche V, Levy R. High‐clearance anti‐amyloid immunotherapies in Alzheimer's disease. Part 1: meta‐analysis and review of efficacy and safety data, and medico‐economical aspects. Rev Neurol (Paris). 2022;178(10):1011‐1030. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

