C5 inhibitor avacincaptad pegol treatment for geographic atrophy: A comprehensive review
- PMID: 39073397
- PMCID: PMC11457614
- DOI: 10.1080/1750743X.2024.2368342
C5 inhibitor avacincaptad pegol treatment for geographic atrophy: A comprehensive review
Abstract
Geographic atrophy (GA) remains a leading cause of central vision loss with no known cure. Until recently, there were no approved treatments for GA, often resulting in poor quality of life for affected patients. GA is characterized by atrophic lesions on the retina that may eventually threaten the fovea. Emerging treatments have demonstrated the ability to reduce the rate of lesion growth, potentially preserving visual function. Avacincaptad pegol (ACP; Astellas Pharma Inc), a complement component 5 inhibitor, is an FDA-approved treatment for GA that has been evaluated in numerous clinical trials. Here we review the current clinical trial landscape of ACP, including critical post hoc analyses that suggest ACP may reduce the risk of severe loss among patients with GA.
Keywords: C5 inhibition; age-related macular degeneration (AMD); aptamer; avacincaptad pegol (ACP); complement; geographic atrophy (GA); retinal disease.
Plain language summary
Geographic atrophy (GA) is an advanced form of eye disease age-related macular degeneration. In people with GA, light-sensitive cells at the back of the eye (the retina) start to die, forming lesions. GA lesions usually get bigger over time and can lead to blindness. New medicines are being studied that work by slowing the growth of GA lesions. Avacincaptad pegol (ACP) is one medicine that acts on the immune system and is designed to block the C5 protein, helping stop the immune system from attacking cells in the retina. Based on clinical studies, ACP was shown to slow the growth of GA over time and has been approved by the FDA. This review article summarizes research on ACP.
Conflict of interest statement
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, stock ownership or options and expert testimony.
Figures

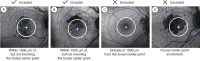
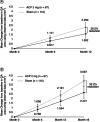

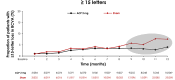

References
-
- Sunness JS. The natural history of geographic atrophy, the advanced atrophic form of age-related macular degeneration. Mol Vis. 1999;5:25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
