The Impact of Autoantibodies on Outcomes in Patients with Idiopathic Pulmonary Fibrosis: Post-Hoc Analyses of the Phase III ASCEND Trial
- PMID: 39073523
- PMCID: PMC11339214
- DOI: 10.1007/s41030-024-00267-x
The Impact of Autoantibodies on Outcomes in Patients with Idiopathic Pulmonary Fibrosis: Post-Hoc Analyses of the Phase III ASCEND Trial
Abstract
Introduction: Clinical practice guidelines recommend autoimmune serological testing in patients newly diagnosed with interstitial lung disease of apparently unknown cause who may have idiopathic pulmonary fibrosis (IPF), in order to exclude connective tissue disease (CTD). Autoantibody positivity has been associated with unique patient profiles and prognosis in patients with IPF who otherwise lack a CTD diagnosis.
Methods: This post-hoc analysis of patients with IPF from the Phase III ASCEND trial (NCT01366209) evaluated the association of antinuclear antibodies (ANA), rheumatoid factor (RF) and anti-cyclic citrullinated peptide (anti-CCP) status with baseline disease characteristics, disease progression [percent predicted forced vital capacity (%FVC), forced vital capacity (FVC) volume and progression-free survival (PFS)], and treatment outcomes with pirfenidone and placebo (%FVC, FVC and PFS).
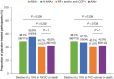
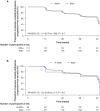
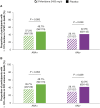
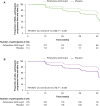
Results: Of 555 participants, 244/514 (47.5%) were ANA positive (ANA+), 83/514 (16.1%) had high ANA+ (ANA titre ≥ 1:160 or positive nucleolar- or centromere-staining patterns), 60/555 (10.8%) were RF positive (RF+) and/or anti-CCP positive (anti-CCP+) and 270/514 (52.5%) were autoantibody negative (AAb-). Baseline demographics and characteristics were generally comparable between autoantibody subgroups. Although not statistically significant, more placebo-treated participants with ANA+ or high ANA+ had a decline from baseline to Week 52 of ≥ 10% in %FVC or death (48.7% and 55.9%, respectively) or in FVC volume or death (48.7% and 47.1%, respectively) compared with the AAb- group (%FVC or death: 42.0%; FVC volume or death: 42.0%). The RF+ and/or anti-CCP+ group was similar to AAb-. No differences were observed in PFS. A treatment benefit for pirfenidone versus placebo was observed regardless of autoantibody status [PFS: ANA+ HR (95% CI): 0.56 (0.37 to 0.86), P = 0.007; AAb- HR (95% CI): 0.50 (0.32 to 0.78), P = 0.002].
Conclusion: IPF disease course did not differ by autoantibody status in ASCEND. Pirfenidone had a treatment benefit regardless of the presence of ANA.
Trial registration: ClinicalTrials.gov identifier, NCT01366209.
Keywords: Anti-cyclic citrullinated peptide (anti-CCP); Antinuclear antibody (ANA); Idiopathic pulmonary fibrosis (IPF); Pirfenidone (PF); Rheumatoid factor (RF).
Plain language summary
People with idiopathic pulmonary fibrosis sometimes have abnormal antibodies, called autoantibodies, in their blood. Uncommonly, autoantibodies may mistakenly target the person’s own tissues, including the lungs. It is unknown whether these autoantibodies cause idiopathic pulmonary fibrosis or make it worse. This analysis looked at data from the ASCEND clinical trial in people with idiopathic pulmonary fibrosis, who were split randomly into two groups to receive tablets of either a medicine called pirfenidone or a placebo for 52 weeks. One goal was to see whether people with certain autoantibodies called antinuclear antibodies (‘ANA’ for short), rheumatoid factor (‘RF’) and anti-cyclic citrullinated peptide (‘anti-CCP’) had different traits from people without autoantibodies, such as age, race or smoking history. Other goals were to see if autoantibodies affected (1) how well people’s lungs worked during the trial, (2) how quickly people’s idiopathic pulmonary fibrosis got worse or they died and (3) how well pirfenidone worked. The analysis showed that most traits were similar in people with and without autoantibodies. In people who received placebo, the change in lung function during the trial was not different for people with ANA, RF or anti-CCP compared with people with no autoantibodies. People who received pirfenidone were less likely to have worsening lung function, or die, than people who received placebo, regardless of whether or not they had autoantibodies. Doctors evaluating patients with idiopathic pulmonary fibrosis should consider the impact of autoantibodies and feel confident that pirfenidone is effective regardless of whether or not autoantibodies are present.
© 2024. The Author(s).
Conflict of interest statement
Tejaswini Kulkarni reports consultancy and speaker fees from Boehringer Ingelheim and consultancy fees from Aileron Therapeutics, PureTech LYT-100 Inc., United Therapeutics Corporation and Veracyte. Chad A. Newton reports consultancy fees from Boehringer Ingelheim. Dr. Newton is supported by the National Heart, Lung, and Blood Institute (K23 HL148498). Sachin Gupta is an employee of Genentech, Inc. Katerina Samara is an employee of F. Hoffmann-La Roche, Ltd. Elana J. Bernstein reports grants, consultancy fees and support for meeting attendance from Boehringer Ingelheim. Dr. Bernstein is also supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant number K23 AR075112), the National Heart, Lung, and Blood Institute (grant number R01 HL164758), and the Department of Defence (grant number W81XWH2210163).
Figures
References
Associated data
LinkOut - more resources
Full Text Sources
Medical

