Proteasome activity inhibition mediates endoplasmic reticulum stress-apoptosis in triptolide/lipopolysaccharide-induced hepatotoxicity
- PMID: 39073694
- PMCID: PMC11286718
- DOI: 10.1007/s10565-024-09903-3
Proteasome activity inhibition mediates endoplasmic reticulum stress-apoptosis in triptolide/lipopolysaccharide-induced hepatotoxicity
Abstract
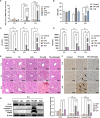
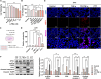
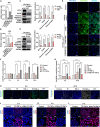
Triptolide (TP) is a major active and toxic composition of the Chinese medicine Tripterygium wilfordii Hook. F. (TWHF), exhibiting various therapeutic bioactivities. Among the toxic effects, the hepatotoxicity of TP deserves serious attention. Previously, our research group proposed a new view of TP-related hepatotoxicity: hepatic hypersensitivity under lipopolysaccharide (LPS) stimulation. However, the mechanism of TP/LPS-induced hepatic hypersensitivity remains unclear. In this study, we investigated the mechanism underlying TP/LPS-induced hypersensitivity from the perspective of the inhibition of proteasome activity, activated endoplasmic reticulum stress (ERS)-related apoptosis, and the accumulation of reactive oxygen species (ROS). Our results showed that N-acetylcysteine (NAC), a common ROS inhibitor, decreased the expression of cleaved caspase-3 and cleaved PARP, which are associated with FLIP enhancement. Moreover, 4-phenylbutyric acid (4-PBA), an ERS inhibitor, was able to alleviate TP/LPS-induced hepatotoxicity by reducing ERS-related apoptosis protein expression (GRP78, p-eIF2α/eIF2α, ATF4, CHOP, cleaved caspase-3 and cleaved PARP) and ROS levels, with ATF4 being an indispensable mediator. In addition, the proteasome activity inhibitor MG-132 further aggravated ERS-related apoptosis, which indicated that the inhibition of proteasome activity also plays an important role in TP/LPS-related liver injuries. In summary, we propose that TP/LPS may upregulate the activation of ERS-associated apoptosis by inhibiting proteasome activity and enhancing ROS production through ATF4.
Keywords: Apoptosis; Endoplasmic reticulum stress; Proteasome activity; Reactive oxygen species; Triptolide.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

