Association of busulfan exposure and outcomes after HCT for patients with an inborn error of immunity
- PMID: 39074263
- PMCID: PMC11470247
- DOI: 10.1182/bloodadvances.2024013275
Association of busulfan exposure and outcomes after HCT for patients with an inborn error of immunity
Abstract
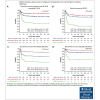
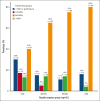
Allogeneic hematopoietic cell transplantation (HCT) is a potentially curative treatment strategy for patients with inborn errors of immunities (IEIs). The objective of this study was to assess the optimal busulfan exposure before allogeneic HCT for patients with an IEI who received an IV busulfan-based conditioning regimen. Patients from 17 international centers were included. The main outcome of interest was event-free survival (EFS). Patients were categorized into 4 IEI subgroups: combined immunodeficiency (CID), severe combined immunodeficiency (SCID), neutrophil disorders, and hemophagocytic lymphohistiocytosis (HLH)-related disorders. Busulfan exposure was calculated by individual centers (area under the curve [AUC]CENTER) and re-estimated using a nonlinear mixed-effects model (NONMEM; exposure defined as AUCNONMEM). Overall, 562 patients were included: 173 (30.8%) with CID, 154 (27.4%) with SCID, 101 (18.0%) with HLH-related disorders, and 134 (23.8%) with neutrophil disorders. The median busulfan AUCNONMEM was 69.0 mg × h/L and correlated poorly with the AUCCENTER (r2 = 0.54). In patients with SCID, HLH-related, and neutrophil disorders with a busulfan AUCNONMEM of 70 to 90 mg × h/L, 2-year EFS was superior to <70 mg × h/L, and >90 mg ×h/L. Full donor chimerism increased with higher busulfan AUCNONMEM, plateauing at 90 mg × h/L. For patients with CID, the optimal AUCNONMEM for donor chimerism was found to be >70 mg × h/L. Improved EFS and higher donor chimerism may be achieved by targeting a cumulative busulfan AUCNONMEM of 80 mg × h/L (range, 70-90). Our study stresses the importance of uniformly using a validated population pharmacokinetic model to estimate AUCNONMEM.
Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution.
Conflict of interest statement
Conflict-of-interest disclosure: S.P. receives support for the conduct of sponsored trials through DFCI/BCH from Atara, AlloVir, and Jasper Therapeutics; consults for Cellevolve and Pierre Fabre; receives honorarium from Regeneron; and reports an IP license to Atara Biotherapeutics with all rights assigned to the Memorial Sloan Kettering Cancer Center. J.-J.B. received honoraria from Sobi, Sanofi, CTI, Smart Immune, Merck, and Advanced Clinical (all not related to this study). C.C.D. is a consultant for Jazz Pharmaceuticals and Alexion, Inc. The remaining authors declare no competing financial interests.
Figures


References
-
- Peshko D, Kulbachinskaya E, Korsunskiy I, et al. Health-related quality of life in children and adults with primary immunodeficiencies: a systematic review and meta-analysis. J Allergy Clin Immunol Pract. 2019;7(6):1929–1957.e5. - PubMed
-
- Bognàr T, Bartelink IH, Egberts TCG, et al. Association between the magnitude of intravenous busulfan exposure and development of hepatic veno-occlusive disease in children and young adults undergoing myeloablative allogeneic hematopoietic cell transplantation. Transplant Cell Ther. 2022;28(4):196–202. - PubMed

