TOWARDS Study: Patient-Derived Xenograft Engraftment Predicts Poor Survival in Patients With Newly Diagnosed Triple-Negative Breast Cancer
- PMID: 39074345
- PMCID: PMC11371112
- DOI: 10.1200/PO.23.00724
TOWARDS Study: Patient-Derived Xenograft Engraftment Predicts Poor Survival in Patients With Newly Diagnosed Triple-Negative Breast Cancer
Abstract
Purpose: Assessing risk of recurrence for nonmetastatic triple-negative breast cancer (TNBC) is a key determinant of therapeutic strategy. The best predictor of recurrence risk is failure to achieve a pathologic complete response after preoperative chemotherapy, but it imperfectly correlates with the definitive end points of relapse-free and overall survival (OS). The inability to accurately predict recurrence has led to increasingly toxic treatment regimens for patients with early-stage TNBC. Better assays for recurrence risk are needed to tailor aggressive therapy for patients who need it and avoid overtreatment and unnecessary toxicity for those at low risk. The purpose of this study was to determine if patient-derived xenograft (PDX) engraftment of newly diagnosed breast tumors can serve as an accurate predictor of recurrence and death from breast cancer.
Methods: This study was a blinded noninterventional trial comprising 80 patients with newly diagnosed, nonmetastatic, estrogen receptor (ER)-negative or ER-low breast cancer.
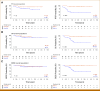
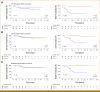
Results: PDX engraftment was strongly associated with relapse in 1 year: 8 of 18 (44.4%) patients whose tumors engrafted relapsed versus 1 of 62 (1.6%) patients whose tumors did not engraft (P < .0001). Patients whose tumors engrafted had a hazard ratio (HR) for relapse of 17.5. HRs for OS and breast cancer-specific survival in PDX+ patients were 21.1 and 39.5, respectively.
Conclusion: We report that the ability of a tumor to engraft as a PDX predicts early recurrence by serving as a functional readout of aggressiveness and prospectively identifies the most devastating tumors. This provides new opportunity to develop surrogate assays, such as biomarkers of engraftment, which will extend the clinical feasibility of this finding.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures

References
-
- Cortazar P, Zhang L, Untch M, et al. : Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 384:164-172, 2014 - PubMed
-
- FDA : Draft guidance for industry: Pathologic complete response in neoadjuvant treatment of high-risk early-stage breast cancer: Use as an endpoint to support accelerated approval 2012. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformati...
-
- Geyer CE, Sikov WM, Huober J, et al. : Long-term efficacy and safety of addition of carboplatin with or without veliparib to standard neoadjuvant chemotherapy in triple-negative breast cancer: 4-year follow-up data from BrighTNess, a randomized phase III trial. Ann Oncol 33:384-394, 2022 - PubMed
-
- Schmid P, Cortes J, Dent R, et al. : Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med 386:556-567, 2022 - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

