Landmark-based auto-contouring of clinical target volumes for radiotherapy of nasopharyngeal cancer
- PMID: 39074490
- PMCID: PMC11492310
- DOI: 10.1002/acm2.14474
Landmark-based auto-contouring of clinical target volumes for radiotherapy of nasopharyngeal cancer
Abstract
Background: The delineation of clinical target volumes (CTVs) for radiotherapy for nasopharyngeal cancer is complex and varies based on the location and extent of disease.
Purpose: The current study aimed to develop an auto-contouring solution following one protocol guidelines (NRG-HN001) that can be adjusted to meet other guidelines, such as RTOG-0225 and the 2018 International guidelines.
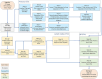
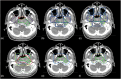
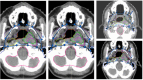
Methods: The study used 2-channel 3-dimensional U-Net and nnU-Net framework to auto-contour 27 normal structures in the head and neck (H&N) region that are used to define CTVs in the protocol. To define the CTV-Expansion (CTV1 and CTV2) and CTV-Overall (the outer envelope of all the CTV contours), we used adjustable morphological geometric landmarks and mimicked physician interpretation of the protocol rules by partially or fully including select anatomic structures. The results were evaluated quantitatively using the dice similarity coefficient (DSC) and mean surface distance (MSD) and qualitatively by independent reviews by two H&N radiation oncologists.
Results: The auto-contouring tool showed high accuracy for nasopharyngeal CTVs. Comparison between auto-contours and clinical contours for 19 patients with cancers of various stages showed a DSC of 0.94 ± 0.02 and MSD of 0.4 ± 0.4 mm for CTV-Expansion and a DSC of 0.83 ± 0.02 and MSD of 2.4 ± 0.5 mm for CTV-Overall. Upon independent review, two H&N physicians found the auto-contours to be usable without edits in 85% and 75% of cases. In 15% of cases, minor edits were required by both physicians. Thus, one physician rated 100% of the auto-contours as usable (use as is, or after minor edits), while the other physician rated 90% as usable. The second physician required major edits in 10% of cases.
Conclusions: The study demonstrates the ability of an auto-contouring tool to reliably delineate nasopharyngeal CTVs based on protocol guidelines. The tool was found to be clinically acceptable by two H&N radiation oncology physicians in at least 90% of the cases.
Keywords: auto‐contouring; clinical target volume; nasopharynx cancer.
© 2024 The Author(s). Journal of Applied Clinical Medical Physics published by Wiley Periodicals LLC on behalf of American Association of Physicists in Medicine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Sung H, Ferlay, J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209‐249. - PubMed
-
- Hunt MA, Zelefsky MJ, Wolden S, et al. Treatment planning and delivery of intensity‐modulated radiation therapy for primary nasopharynx cancer. Int J Radiat Oncol. 2001;49:623‐632. - PubMed
-
- Lee AW, Ng WT, Pan JJ, et al. International guideline for the delineation of the clinical target volumes (CTV) for nasopharyngeal carcinoma. Radiother Oncol. 2018;126:25‐36. - PubMed
-
- Hansen CR, Johansen J, Samsøe E, et al. Consequences of introducing geometric GTV to CTV margin expansion in DAHANCA contouring guidelines for head and neck radiotherapy. Radiother Oncol. 2018;126:43‐47. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

