Sleep Consolidation Potentiates Sensorimotor Adaptation
- PMID: 39074983
- PMCID: PMC11376339
- DOI: 10.1523/JNEUROSCI.0325-24.2024
Sleep Consolidation Potentiates Sensorimotor Adaptation
Abstract
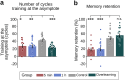
Contrary to its well-established role in declarative learning, the impact of sleep on motor memory consolidation remains a subject of debate. Current literature suggests that while motor skill learning benefits from sleep, consolidation of sensorimotor adaptation (SMA) depends solely on the passage of time. This has led to the proposal that SMA may be an exception to other types of memories. Here, we addressed this ongoing controversy in humans through three comprehensive experiments using the visuomotor adaptation paradigm (N = 290, 150 females). In Experiment 1, we investigated the impact of sleep on memory retention when the temporal gap between training and sleep was not controlled. In line with the previous literature, we found that memory consolidates with the passage of time. In Experiment 2, we used an anterograde interference protocol to determine the time window during which SMA memory is most fragile and, thus, potentially most sensitive to sleep intervention. Our results show that memory is most vulnerable during the initial hour post-training. Building on this insight, in Experiment 3, we investigated the impact of sleep when it coincided with the critical first hour of memory consolidation. This manipulation unveiled a benefit of sleep (30% memory enhancement) alongside an increase in spindle density and spindle-SO coupling during NREM sleep, two well-established neural markers of sleep consolidation. Our findings reconcile seemingly conflicting perspectives on the active role of sleep in motor learning and point to common mechanisms at the basis of memory formation.
Keywords: EEG; consolidation; human; motor learning; sleep.
Copyright © 2024 the authors.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous
