Forskolin reverses the O-GlcNAcylation dependent decrease in GABAAR current amplitude at hippocampal synapses possibly at a neurosteroid site on GABAARs
- PMID: 39075105
- PMCID: PMC11286967
- DOI: 10.1038/s41598-024-66025-w
Forskolin reverses the O-GlcNAcylation dependent decrease in GABAAR current amplitude at hippocampal synapses possibly at a neurosteroid site on GABAARs
Abstract
GABAergic transmission is influenced by post-translational modifications, like phosphorylation, impacting channel conductance, allosteric modulator sensitivity, and membrane trafficking. O-GlcNAcylation is a post-translational modification involving the O-linked attachment of β-N-acetylglucosamine on serine/threonine residues. Previously we reported an acute increase in O-GlcNAcylation elicits a long-term depression of evoked GABAAR inhibitory postsynaptic currents (eIPSCs) onto hippocampal principal cells. Importantly, O-GlcNAcylation and phosphorylation can co-occur or compete for the same residue; whether they interact in modulating GABAergic IPSCs is unknown. We tested this by recording IPSCs from hippocampal principal cells and pharmacologically increased O-GlcNAcylation, before or after increasing serine phosphorylation using the adenylate cyclase activator, forskolin. Although forskolin had no significant effect on baseline eIPSC amplitude, we found that a prior increase in O-GlcNAcylation unmasks a forskolin-dependent increase in eIPSC amplitude, reversing the O-GlcNAc-induced eIPSC depression. Inhibition of adenylate cyclase or protein kinase A did not prevent the potentiating effect of forskolin, indicating serine phosphorylation is not the mechanism. Surprisingly, increasing O-GlcNAcylation also unmasked a potentiating effect of the neurosteroids 5α-pregnane-3α,21-diol-20-one (THDOC) and progesterone on eIPSC amplitude in about half of the recorded cells, mimicking forskolin. Our findings show that under conditions of heightened O-GlcNAcylation, the neurosteroid site on synaptic GABAARs is possibly accessible to agonists, permitting strengthening of synaptic inhibition.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
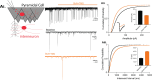

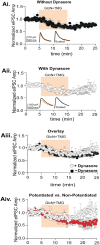



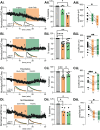
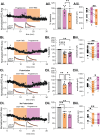
Update of
-
Forskolin reverses the O-GlcNAcylation dependent decrease in GABAAR current amplitude at hippocampal synapses possibly through a neurosteroid site on GABAARs.bioRxiv [Preprint]. 2024 Mar 21:2024.03.06.583612. doi: 10.1101/2024.03.06.583612. bioRxiv. 2024. Update in: Sci Rep. 2024 Jul 29;14(1):17461. doi: 10.1038/s41598-024-66025-w. PMID: 38496430 Free PMC article. Updated. Preprint.
-
Forskolin reverses the O-GlcNAcylation dependent decrease in GABAAR current amplitude at hippocampal synapses possibly at a neurosteroid site on GABAARs.Res Sq [Preprint]. 2024 Apr 10:rs.3.rs-4140038. doi: 10.21203/rs.3.rs-4140038/v1. Res Sq. 2024. Update in: Sci Rep. 2024 Jul 29;14(1):17461. doi: 10.1038/s41598-024-66025-w. PMID: 38659738 Free PMC article. Updated. Preprint.
Similar articles
-
Forskolin reverses the O-GlcNAcylation dependent decrease in GABAAR current amplitude at hippocampal synapses possibly through a neurosteroid site on GABAARs.bioRxiv [Preprint]. 2024 Mar 21:2024.03.06.583612. doi: 10.1101/2024.03.06.583612. bioRxiv. 2024. Update in: Sci Rep. 2024 Jul 29;14(1):17461. doi: 10.1038/s41598-024-66025-w. PMID: 38496430 Free PMC article. Updated. Preprint.
-
Forskolin reverses the O-GlcNAcylation dependent decrease in GABAAR current amplitude at hippocampal synapses possibly at a neurosteroid site on GABAARs.Res Sq [Preprint]. 2024 Apr 10:rs.3.rs-4140038. doi: 10.21203/rs.3.rs-4140038/v1. Res Sq. 2024. Update in: Sci Rep. 2024 Jul 29;14(1):17461. doi: 10.1038/s41598-024-66025-w. PMID: 38659738 Free PMC article. Updated. Preprint.
-
Acute Increases in Protein O-GlcNAcylation Dampen Epileptiform Activity in Hippocampus.J Neurosci. 2017 Aug 23;37(34):8207-8215. doi: 10.1523/JNEUROSCI.0173-16.2017. Epub 2017 Jul 31. J Neurosci. 2017. PMID: 28760863 Free PMC article.
-
Phosphorylation of GABAA receptors influences receptor trafficking and neurosteroid actions.Psychopharmacology (Berl). 2014 Sep;231(17):3453-65. doi: 10.1007/s00213-014-3617-z. Epub 2014 May 22. Psychopharmacology (Berl). 2014. PMID: 24847959 Free PMC article. Review.
-
Roles of O-GlcNAcylation on amyloid-β precursor protein processing, tau phosphorylation, and hippocampal synapses dysfunction in Alzheimer's disease.Neurol Res. 2016 Feb;38(2):177-86. doi: 10.1080/01616412.2015.1133485. Epub 2016 Mar 14. Neurol Res. 2016. PMID: 27078700 Review.
Cited by
-
O-GlcNAcylation in Endocrinology: The Sweet Link.Endocrinology. 2025 Apr 22;166(6):bqaf072. doi: 10.1210/endocr/bqaf072. Endocrinology. 2025. PMID: 40209111 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

