Caspase-4 in glioma indicates deterioration and unfavorable prognosis by affecting tumor cell proliferation and immune cell recruitment
- PMID: 39075190
- PMCID: PMC11286837
- DOI: 10.1038/s41598-024-65018-z
Caspase-4 in glioma indicates deterioration and unfavorable prognosis by affecting tumor cell proliferation and immune cell recruitment
Abstract
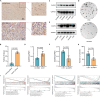
Gliomas are the most common malignant tumors of the central nervous system, accounting for approximately 80% of all malignant brain tumors. Accumulating evidence suggest that pyroptosis plays an essential role in the progression of cancer. Unfortunately, the effect of the pyroptosis-related factor caspase-4 (CASP4) on immunotherapy and drug therapy for tumors has not been comprehensively investigated. In this study, we systematically screened six hub genes by pooling differential pyroptosis-related genes in The Cancer Genome Atlas (TCGA) glioma data and the degree of centrality of index-related genes in the protein-protein interaction network. We performed functional and pathway enrichment analyses of the six hub genes to explore their biological functions and potential molecular mechanisms. We then investigated the importance of CASP4 using Kaplan-Meier survival analysis of glioma patients. TCGA and the Chinese Glioma Genome Atlas (CGGA) databases showed that reduced CASP4 expression leads to the potent clinical deterioration of glioma patients. Computational analysis of the effect of CASP4 on the infiltration level and recruitment of glioma immune cells revealed that CASP4 expression was closely associated with a series of tumor-suppressive immune checkpoint molecules, chemokines, and chemokine receptors. We also found that aberrant CASP4 expression correlated with chemotherapeutic drug sensitivity. Finally, analysis at the cellular and tissue levels indicated an increase in CASP4 expression in glioma, and that CASP4 inhibition significantly inhibited the proliferation of glioma cells. Thus, CASP4 is implicated as a new prognostic biomarker for gliomas with the potential to further guide immunotherapy and chemotherapy strategies for glioma patients.
Keywords: CASP4; Chemotherapy; Glioma; Immunotherapy; Pyroptosis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures









References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

