The impact of a digital guideline version on schizophrenia guideline knowledge: results from a multicenter cluster-randomized controlled trial
- PMID: 39075458
- PMCID: PMC11287881
- DOI: 10.1186/s12916-024-03533-6
The impact of a digital guideline version on schizophrenia guideline knowledge: results from a multicenter cluster-randomized controlled trial
Abstract
Background: Clinical practice guidelines are crucial for enhancing healthcare quality and patient outcomes. Yet, their implementation remains inconsistent across various professions and disciplines. Previous findings on the implementation of the German guideline for schizophrenia (2019) revealed low adherence rates among healthcare professionals. Barriers to guideline adherence are multifaceted, influenced by individual, contextual, and guideline-related factors. This study aims to investigate the effectiveness of a digital guideline version compared to print/PDF formats in enhancing guideline adherence.
Methods: A multicenter, cluster-randomized controlled trial was conducted in South Bavaria, Germany, involving psychologists and physicians. Participants were divided into two groups: implementation of the guideline using a digital online version via the MAGICapp platform and the other using the traditional print/PDF version. The study included a baseline assessment and a post-intervention assessment following a 6-month intervention phase. The primary outcome was guideline knowledge, which was assessed using a guideline knowledge questionnaire.
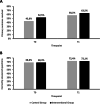
Results: The study included 217 participants at baseline and 120 at post-intervention. Both groups showed significant improvements in guideline knowledge; however, no notable difference was found between both study groups regarding guideline knowledge at either time points. At baseline, 43.6% in the control group (CG) and 52.5% of the interventional group (IG) met the criterion. There was no significant difference in the primary outcome between the two groups at either time point (T0: Chi2(1) = 1.65, p = 0.199, T1: Chi2(1) = 0.34, p = 0.561). At post-intervention, both groups improved, with 58.2% in the CG and 63.5% in the IG meeting this criterion.
Conclusions: While the study did not include a control group without any implementation strategy, the overall improvement in guideline knowledge following an implementation strategy, independent of the format, was confirmed. The digital guideline version, while not superior in enhancing knowledge, showed potential benefits in shared decision-making skills. However, familiarity with traditional formats and various barriers to digital application may have influenced these results. The study highlights the importance of tailored implementation strategies, especially for younger healthcare providers.
Trial registration: https://drks.de/search/de/trial/DRKS00028895.
Keywords: Cluster-randomized controlled trial; Guideline implementation; Health personnel; MAGICapp; Practice guideline.
© 2024. The Author(s).
Conflict of interest statement
A.H. was a member of advisory boards and received paid speakership by Boehringer-Ingelheim, Lundbeck, Otsuka, Rovi, and Recordati. He received paid speakership by AbbVie and Advanz. He is editor of the AWMF German guidelines for schizophrenia. M.R. was a member of advisory board and received paid speakership by Rovi. K.F. has received travel grants for congress participation by Janssen; he is vice chairman of ackpa (Association of Chief Psychiatrists in German General Hospitals) and Executive Committee Member of the DGPPN (German Association for Psychiatry, Psychotherapy and Psychosomatics). P.F. is co-editor of the German (DGPPN) schizophrenia treatment guidelines, co-author of the WFSBP schizophrenia treatment guidelines; on advisory boards and speaker fees from Janssen, Lundbeck, Otsuka, Servier, and Richter. In the last three years. S.L.2 has received honoraria for advising/consulting and/or for lectures and/or for educational material from Angelini, Boehringer Ingelheim, Eisai, Ekademia, GedeonRichter, Janssen, Karuna, Kynexis, Lundbeck, Medichem, Medscape, Mitsubishi, Neurotorium, Otsuka, NovoNordisk, Recordati, Rovi, Teva. Over the past three years, S.H. has received lecture fees from Johnson & Johnson, Boehringer-Ingelheim, Recordati and Otsuka/Lundbeck, as well as fees for scientific advisory boards in clinical trials for TEVA, ROVI, Merck and Boehringer-Ingelheim. All other authors do not report any conflicts of interest related to the subject of this work.
Figures
References
-
- Field MJ, Lohr KN, editors. Clinical practice guidelines: directions for a new program. Washington (DC): National Academies Press (US); 1990. - PubMed
-
- Gaigl G, Täumer E, Merz K, Zöscher S, Wagner S, Kösters M, et al. Multifactorial barriers in the implementation of schizophrenia and psychosocial therapies guidelines: a quantitative study across different professions. Schizophr Res. 2021;228:425–34. Available from: https://pubmed.ncbi.nlm.nih.gov/33561620/. 10.1016/j.schres.2021.01.010 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous