Plancitoxin-1 mediates extracellular trap evasion by the parasitic helminth Trichinella spiralis
- PMID: 39075478
- PMCID: PMC11287892
- DOI: 10.1186/s12915-024-01958-2
Plancitoxin-1 mediates extracellular trap evasion by the parasitic helminth Trichinella spiralis
Erratum in
-
Author Correction: Plancitoxin-1 mediates extracellular trap evasion by the parasitic helminth Trichinella spiralis.BMC Biol. 2024 Sep 27;22(1):213. doi: 10.1186/s12915-024-02016-7. BMC Biol. 2024. PMID: 39334469 Free PMC article. No abstract available.
Abstract
Background: Trichinella spiralis (T. spiralis) is a parasitic helminth that causes a globally prevalent neglected zoonotic disease, and worms at different developmental stages (muscle larvae, adult worms, newborn larvae) induce immune attack at different infection sites, causing serious harm to host health. Several innate immune cells release extracellular traps (ETs) to entrap and kill most pathogens that invade the body. In response, some unicellular pathogens have evolved a strategy to escape capture by ETs through the secretion of nucleases, but few related studies have investigated multicellular helminths.
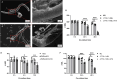
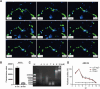
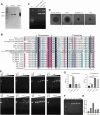
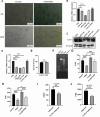
Results: In the present study, we observed that ETs from neutrophils capture adult worms of T. spiralis, while ETs from macrophages trap muscle larvae and newborn larvae, and ETs had a killing effect on parasites in vitro. To defend against this immune attack, T. spiralis secretes plancitoxin-1, a DNase II-like protein, to degrade ETs and escape capture, which is essential for the survival of T. spiralis in the host.
Conclusions: In summary, these findings demonstrate that T. spiralis escapes ET-mediated capture by secreting deoxyribonuclease as a potential conserved immune evasion mechanism, and plancitoxin-1 could be used as a potential vaccine candidate.
Keywords: Trichinella spiralis; Extracellular traps; Immune evasion; Nuclease; Vaccine candidate.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Trichinella spiralis Calreticulin S-Domain Binds to Human Complement C1q to Interfere With C1q-Mediated Immune Functions.Front Immunol. 2020 Nov 19;11:572326. doi: 10.3389/fimmu.2020.572326. eCollection 2020. Front Immunol. 2020. PMID: 33329535 Free PMC article.
-
Characterisation of a plancitoxin-1-like DNase II gene in Trichinella spiralis.PLoS Negl Trop Dis. 2014 Aug 28;8(8):e3097. doi: 10.1371/journal.pntd.0003097. eCollection 2014 Aug. PLoS Negl Trop Dis. 2014. PMID: 25165857 Free PMC article.
-
Molecular characterization of a 31 kDa protein from Trichinella spiralis and its induced immune protection in BALB/c mice.Parasit Vectors. 2018 Dec 5;11(1):625. doi: 10.1186/s13071-018-3198-5. Parasit Vectors. 2018. PMID: 30518426 Free PMC article.
-
Immune responses in mice vaccinated with a DNA vaccine expressing serine protease-like protein from the new-born larval stage of Trichinella spiralis.Parasitology. 2017 May;144(6):712-719. doi: 10.1017/S0031182016002493. Epub 2017 Jan 10. Parasitology. 2017. PMID: 28069101 Free PMC article.
-
Therapeutic potentials of Trichinella spiralis in immune disorders: From allergy to autoimmunity.Parasites Hosts Dis. 2025 May;63(2):123-134. doi: 10.3347/PHD.24086. Epub 2025 May 26. Parasites Hosts Dis. 2025. PMID: 40452274 Free PMC article. Review.
Cited by
-
Author Correction: Plancitoxin-1 mediates extracellular trap evasion by the parasitic helminth Trichinella spiralis.BMC Biol. 2024 Sep 27;22(1):213. doi: 10.1186/s12915-024-02016-7. BMC Biol. 2024. PMID: 39334469 Free PMC article. No abstract available.
References
-
- Bai Y, Ma KN, Sun XY, Dan Liu R, Long SR, Jiang P, Wang ZQ, Cui J. Molecular characterization of a novel cathepsin L from Trichinella spiralis and its participation in invasion, development and reproduction. Acta Trop. 2021;224:106112. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

