Insulin promotes the bone formation capability of human dental pulp stem cells through attenuating the IIS/PI3K/AKT/mTOR pathway axis
- PMID: 39075596
- PMCID: PMC11287875
- DOI: 10.1186/s13287-024-03843-9
Insulin promotes the bone formation capability of human dental pulp stem cells through attenuating the IIS/PI3K/AKT/mTOR pathway axis
Abstract
Background: Insulin has been known to regulate bone metabolism, yet its specific molecular mechanisms during the proliferation and osteogenic differentiation of dental pulp stem cells (DPSCs) remain poorly understood. This study aimed to explore the effects of insulin on the bone formation capability of human DPSCs and to elucidate the underlying mechanisms.
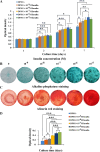
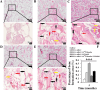
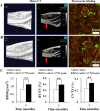
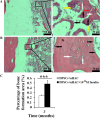
Methods: Cell proliferation was assessed using a CCK-8 assay. Cell phenotype was analyzed by flow cytometry. Colony-forming unit-fibroblast ability and multilineage differentiation potential were evaluated using Toluidine blue, Oil red O, Alizarin red, and Alcian blue staining. Gene and protein expressions were quantified by real-time quantitative polymerase chain reaction and Western blotting, respectively. Bone metabolism and biochemical markers were analyzed using electrochemical luminescence and chemical colorimetry. Cell adhesion and growth on nano-hydroxyapatite/collagen (nHAC) were observed with a scanning electron microscope. Bone regeneration was assessed using micro-CT, fluorescent labeling, immunohistochemical and hematoxylin and eosin staining.
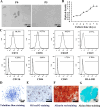
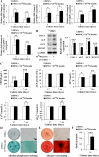
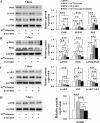
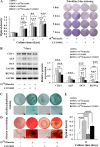
Results: Insulin enhanced the proliferation of human DPSCs as well as promoted mineralized matrix formation in a concentration-dependent manner. 10- 6 M insulin significantly up-regulated osteogenic differentiation-related genes and proteins markedly increased the secretion of bone metabolism and biochemical markers, and obviously stimulated mineralized matrix formation. However, it also significantly inhibited the expression of genes and proteins of receptors and receptor substrates associated with insulin/insulin-like growth factor-1 signaling (IIS) pathway, obviously reduced the expression of the phosphorylated PI3K and the ratios of the phosphorylated PI3K/total PI3K, and notably increased the expression of the total PI3K, phosphorylated AKT, total AKT and mTOR. The inhibitor LY294002 attenuated the responsiveness of 10- 6 M insulin to IIS/PI3K/AKT/mTOR pathway axis, suppressing the promoting effect of insulin on cell proliferation, osteogenic differentiation and bone formation. Implantation of 10- 6 M insulin treated DPSCs into the backs of severe combined immunodeficient mice and the rabbit jawbone defects resulted in enhanced bone formation.
Conclusions: Insulin induces insulin resistance in human DPSCs and effectively promotes their proliferation, osteogenic differentiation and bone formation capability through gradually inducing the down-regulation of IIS/PI3K/AKT/mTOR pathway axis under insulin resistant states.
Keywords: Bone regeneration; Human dental pulp stem cells; Insulin; Insulin/insulin-like growth factor-1 signaling (IIS) pathway; PI3K/AKT/mTOR pathway.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures




References
-
- Ahn JJ, Shin HI. Bone tissue formation in extraction sockets from sites with advanced periodontal disease: a histomorphometric study in humans. Int J Oral Maxillofac Implants. 2008;23:1133–8. - PubMed
-
- Li Q, Wang H, Liu L, Weng Y, Xu S, Li L, Wang Z. Suppression of the NLRP3 inflammasome through activation of the transient receptor potential channel melastatin 2 promotes osteogenesis in tooth extraction sockets of periodontitis. Am J Pathol. 2023;193:213–32. 10.1016/j.ajpath.2022.10.009 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

