Upadacitinib monotherapy versus methotrexate monotherapy in patients with rheumatoid arthritis: efficacy and safety through 5 years in the SELECT-EARLY randomized controlled trial
- PMID: 39075620
- PMCID: PMC11285135
- DOI: 10.1186/s13075-024-03358-x
Upadacitinib monotherapy versus methotrexate monotherapy in patients with rheumatoid arthritis: efficacy and safety through 5 years in the SELECT-EARLY randomized controlled trial
Abstract
Background: To evaluate the efficacy and safety of upadacitinib monotherapy versus methotrexate (MTX) monotherapy over 5 years among MTX-naïve patients with moderately to severely active rheumatoid arthritis (RA) in the long-term extension (LTE) of the phase 3 SELECT-EARLY trial.
Methods: Patients were randomized to receive upadacitinib 15 mg or 30 mg or MTX. Patients who did not achieve CDAI remission and had < 20% improvement in tender and swollen joint counts at week 26 received rescue therapy (addition of MTX in the upadacitinib group and addition of upadacitinib in the MTX group). Efficacy assessments were evaluated over 5 years and are reported as observed (AO) for patients who received continuous monotherapy with upadacitinib 15/30 mg or MTX and by randomized group applying non-responder imputation (NRI). Treatment-emergent adverse events (TEAEs) per 100 patient-years were summarized over 5 years.
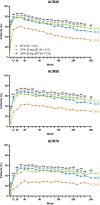
Results: Of 945 patients randomized and treated, 775 (82%) completed week 48 and entered the LTE on study drug. Higher proportions of patients consistently achieved disease activity targets over 5 years with upadacitinib than MTX. In AO analyses, 53%/59% of patients attained CDAI remission with upadacitinib 15/30 mg versus 43% with MTX at week 260. NRI analyses showed better CDAI, DAS28(CRP), and ACR responses with upadacitinib relative to MTX at week 260 (all comparisons, nominal P < .001). Upadacitinib treatment also resulted in numerically greater inhibition of structural joint progression through week 260 compared to MTX. Most TEAEs, serious AEs, and AEs leading to discontinuation were numerically higher in patients receiving upadacitinib 30 mg. Rates of serious infections, herpes zoster, creatine phosphokinase elevation, nonmelanoma skin cancer, and neutropenia were numerically higher with upadacitinib than MTX. The observed safety profile of upadacitinib over 5 years was consistent with earlier trial results and integrated phase 3 safety analyses.
Conclusions: Upadacitinib showed better clinical responses versus MTX in patients with RA throughout the 5-year trial. Higher rates of several AEs were observed with upadacitinib, especially in the 30 mg group, compared to MTX. When used as monotherapy in MTX-naïve patients, the approved upadacitinib 15 mg dose showed better long-term efficacy versus MTX and an overall favorable benefit-risk profile.
Trial registration: NCT02706873.
Keywords: JAK inhibitor; Long-term extension; Methotrexate; Randomized controlled trial; Rheumatoid arthritis; SELECT-EARLY; Targeted synthetic DMARD; Upadacitinib.
© 2024. The Author(s).
Conflict of interest statement
R van Vollenhoven: Research Support (institutional grants): BMS, UCB; support for educational programs (institutional grants): AstraZeneca, Galapagos, MSD, Novartis, Pfizer, Roche, Sanofi, UCB; consultancy, for which institutional and/or personal honoraria were received: AbbVie, AstraZeneca, Biogen, BMS, Galapagos, Janssen, Pfizer, RemeGen, UCB; speaker, for which institutional and/or personal honoraria were received: AbbVie, AstraZeneca, BMS, Galapagos, GSK, Janssen, Pfizer, UCB.V Strand: Consultant for AbbVie, Alpine Immune Sciences, Alumis, Amgen, AstraZeneca, Bayer, Blackrock, BMS, Boehringer Ingelheim, Celltrion, Ermium, Genentech/Roche, GlaxoSmithKline, Horizon, Inmedix, Janssen, Kiniksa, Lilly, Merck, MiMedx, Novartis, Omeros, Pfizer, R-Pharm, Regeneron, Samsung, Sandoz, Sanofi, Scipher, Setpoint, Sorrento, Spherix, and Urica.T Takeuchi: Grant/research support from AbbVie and Eisai; consulting fees from AbbVie, Astellas Pharma, Eli Lilly Japan, and Gilead Sciences; speaker/honoraria from AbbVie, Astellas Pharma, Eisai, Eli Lilly Japan, Gilead Sciences, and Pfizer Japan.J Aelion: Grant or research support: AbbVie, Acceleron, Acelyrin, Aclaris Therapeutics, Alpine Immune Sciences, Amgen, AstraZeneca, Biogen, BMS, Eli Lilly, Galapagos, GSK, Horizon, Janssen, Novartis, Selecta, UCB, Ventyx; consultancy for AbbVie, Amgen, BMS, Janssen, Novartis; speaker for AbbVie and Amgen. N Chávez: Speakers bureau for AbbVie, Janssen, and Pfizer; consultant of AbbVie, Janssen, and Pfizer; grant/research support from AbbVie, Galapagos, Gilead, Pfizer, and Sanofi. P Mannucci Walter: consulting fees from AbbVie; grant/research support from AbbVie, Bristol-Myers Squibb, Lilly, Genentech/Roche, GSK, Janssen, and UCB. Atul Singhal: No conflicts of interest to declare. J Swierkot: Speaker, research grants, and consulting fees from AbbVie, Amgen, Astra Zeneca, Celltrion, Eli Lilly, Novartis, Sandoz, Pfizer, Roche, BMS, UCB, MSD, Accord, and Janssen.N Khan, X Bu, Y Li, SK Penn, HS Camp: Employee of AbbVie and may hold stock or options.
Figures






References
-
- Pincus T, Gibson KA, Castrejon I. Update on methotrexate as the anchor drug for rheumatoid arthritis. Bull Hosp Jt Dis (2013) 2013;71 Suppl 1:S9-19. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

