Stiffness-tunable biomaterials provide a good extracellular matrix environment for axon growth and regeneration
- PMID: 39075897
- PMCID: PMC11624885
- DOI: 10.4103/NRR.NRR-D-23-01874
Stiffness-tunable biomaterials provide a good extracellular matrix environment for axon growth and regeneration
Abstract
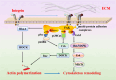
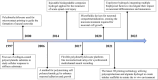
Neuronal growth, extension, branching, and formation of neural networks are markedly influenced by the extracellular matrix-a complex network composed of proteins and carbohydrates secreted by cells. In addition to providing physical support for cells, the extracellular matrix also conveys critical mechanical stiffness cues. During the development of the nervous system, extracellular matrix stiffness plays a central role in guiding neuronal growth, particularly in the context of axonal extension, which is crucial for the formation of neural networks. In neural tissue engineering, manipulation of biomaterial stiffness is a promising strategy to provide a permissive environment for the repair and regeneration of injured nervous tissue. Recent research has fine-tuned synthetic biomaterials to fabricate scaffolds that closely replicate the stiffness profiles observed in the nervous system. In this review, we highlight the molecular mechanisms by which extracellular matrix stiffness regulates axonal growth and regeneration. We highlight the progress made in the development of stiffness-tunable biomaterials to emulate in vivo extracellular matrix environments, with an emphasis on their application in neural repair and regeneration, along with a discussion of the current limitations and future prospects. The exploration and optimization of the stiffness-tunable biomaterials has the potential to markedly advance the development of neural tissue engineering.
Copyright © 2025 Copyright: © 2025 Neural Regeneration Research.
Conflict of interest statement
Figures


Similar articles
-
Transplantable living scaffolds comprised of micro-tissue engineered aligned astrocyte networks to facilitate central nervous system regeneration.Acta Biomater. 2016 Jul 1;38:44-58. doi: 10.1016/j.actbio.2016.04.021. Epub 2016 Apr 29. Acta Biomater. 2016. PMID: 27090594 Free PMC article.
-
A State-of-the-Art of Functional Scaffolds for 3D Nervous Tissue Regeneration.Front Bioeng Biotechnol. 2021 Mar 18;9:639765. doi: 10.3389/fbioe.2021.639765. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 33816451 Free PMC article. Review.
-
Biomimetic Silk Scaffolds with an Amorphous Structure for Soft Tissue Engineering.ACS Appl Mater Interfaces. 2018 Mar 21;10(11):9290-9300. doi: 10.1021/acsami.7b19204. Epub 2018 Mar 8. ACS Appl Mater Interfaces. 2018. PMID: 29485270
-
Substrate stiffness affects neural network activity in an extracellular matrix proteins dependent manner.Colloids Surf B Biointerfaces. 2018 Oct 1;170:729-735. doi: 10.1016/j.colsurfb.2018.03.042. Epub 2018 Mar 27. Colloids Surf B Biointerfaces. 2018. PMID: 30005410
-
Recent Trends in the Development of Bone Regenerative Biomaterials.Front Cell Dev Biol. 2021 May 7;9:665813. doi: 10.3389/fcell.2021.665813. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34026758 Free PMC article. Review.
Cited by
-
Sandwich-Layered Structure Nanofiber Conduits Facilitating the Repair of Long-Gap Nerve Defects.ACS Omega. 2025 Jun 5;10(23):24961-24972. doi: 10.1021/acsomega.5c02439. eCollection 2025 Jun 17. ACS Omega. 2025. PMID: 40547633 Free PMC article.
-
Investigation of Persistent Photoconductivity of Gallium Nitride Semiconductor and Differentiation of Primary Neural Stem Cells.Molecules. 2024 Sep 19;29(18):4439. doi: 10.3390/molecules29184439. Molecules. 2024. PMID: 39339434 Free PMC article.
-
The influence of hydrogel stiffness on axonal regeneration after spinal cord injury.PLoS One. 2025 Jun 25;20(6):e0325798. doi: 10.1371/journal.pone.0325798. eCollection 2025. PLoS One. 2025. PMID: 40560940 Free PMC article.
References
-
- Anderson L, Burnstein RM, He X, Luce R, Furlong R, Foltynie T, Sykacek P, Menon DK, Caldwell MA. Gene expression changes in long term expanded human neural progenitor cells passaged by chopping lead to loss of neurogenic potential in vivo. Exp Neurol. 2007;204:512–524. - PubMed
-
- Aoun L, Weiss P, Laborde A, Ducommun B, Lobjois V, Vieu C. Microdevice arrays of high aspect ratio poly(dimethylsiloxane) pillars for the investigation of multicellular tumour spheroid mechanical properties. Lab Chip. 2014;14:2344–2353. - PubMed
LinkOut - more resources
Full Text Sources

