Endoplasmic reticulum stress and autophagy in cerebral ischemia/reperfusion injury: PERK as a potential target for intervention
- PMID: 39075912
- PMCID: PMC11624856
- DOI: 10.4103/NRR.NRR-D-23-00794
Endoplasmic reticulum stress and autophagy in cerebral ischemia/reperfusion injury: PERK as a potential target for intervention
Abstract
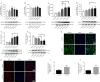
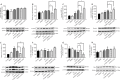
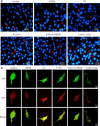
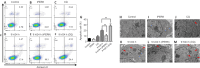
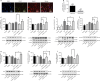
JOURNAL/nrgr/04.03/01300535-202505000-00028/figure1/v/2024-07-28T173839Z/r/image-tiff Several studies have shown that activation of unfolded protein response and endoplasmic reticulum (ER) stress plays a crucial role in severe cerebral ischemia/reperfusion injury. Autophagy occurs within hours after cerebral ischemia, but the relationship between ER stress and autophagy remains unclear. In this study, we established experimental models using oxygen-glucose deprivation/reoxygenation in PC12 cells and primary neurons to simulate cerebral ischemia/reperfusion injury. We found that prolongation of oxygen-glucose deprivation activated the ER stress pathway protein kinase-like endoplasmic reticulum kinase (PERK)/eukaryotic translation initiation factor 2 subunit alpha (eIF2α)-activating transcription factor 4 (ATF4)-C/EBP homologous protein (CHOP), increased neuronal apoptosis, and induced autophagy. Furthermore, inhibition of ER stress using inhibitors or by siRNA knockdown of the PERK gene significantly attenuated excessive autophagy and neuronal apoptosis, indicating an interaction between autophagy and ER stress and suggesting PERK as an essential target for regulating autophagy. Blocking autophagy with chloroquine exacerbated ER stress-induced apoptosis, indicating that normal levels of autophagy play a protective role in neuronal injury following cerebral ischemia/reperfusion injury. Findings from this study indicate that cerebral ischemia/reperfusion injury can trigger neuronal ER stress and promote autophagy, and suggest that PERK is a possible target for inhibiting excessive autophagy in cerebral ischemia/reperfusion injury.
Copyright © 2025 Copyright: © 2025 Neural Regeneration Research.
Conflict of interest statement
Figures

References
-
- Abolhasanpour N, Alihosseini S, Golipourkhalili S, Badalzadeh R, Mahmoudi J, Hosseini L. Effect of melatonin on endoplasmic reticulum-mitochondrial crosstalk in stroke. Arch Med Res. 2021;52:673–682. - PubMed
-
- Allen CL, Bayraktutan U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int J Stroke. 2009;4:461–470. - PubMed
-
- Badiola N, Penas C, Miñano-Molina A, Barneda-Zahonero B, Fadó R, Sánchez-Opazo G, Comella JX, Sabriá J, Zhu C, Blomgren K, Casas C, Rodríguez-Alvarez J. Induction of ER stress in response to oxygen-glucose deprivation of cortical cultures involves the activation of the PERK and IRE-1 pathways and of caspase-12. Cell Death Dis. 2011;2:e149. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

