cUMP elicits interendothelial gap formation during Pseudomonas aeruginosa infection
- PMID: 39076085
- PMCID: PMC11444506
- DOI: 10.1152/ajplung.00164.2023
cUMP elicits interendothelial gap formation during Pseudomonas aeruginosa infection
Abstract
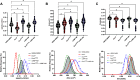
Pseudomonas aeruginosa utilizes a type 3 secretion system to intoxicate host cells with the nucleotidyl cyclase ExoY. After activation by its host cell cofactor, filamentous actin, ExoY produces purine and pyrimidine cyclic nucleotides, including cAMP, cGMP, and cUMP. ExoY-generated cyclic nucleotides promote interendothelial gap formation, impair motility, and arrest cell growth. The disruptive activities of cAMP and cGMP during the P. aeruginosa infection are established; however, little is known about the function of cUMP. Here, we tested the hypothesis that cUMP contributes to endothelial cell barrier disruption during P. aeruginosa infection. Using a membrane permeable cUMP analog, cUMP-AM, we revealed that during infection with catalytically inactive ExoY, cUMP promotes interendothelial gap formation in cultured pulmonary microvascular endothelial cells (PMVECs) and contributes to increased filtration coefficient in the isolated perfused lung. These findings indicate that cUMP contributes to endothelial permeability during P. aeruginosa lung infection.NEW & NOTEWORTHY During pneumonia, bacteria utilize a virulence arsenal to communicate with host cells. The Pseudomonas aeruginosa T3SS directly introduces virulence molecules into the host cell cytoplasm. These molecules are enzymes that trigger interkingdom communication. One of the exoenzymes is a nucleotidyl cyclase that produces noncanonical cyclic nucleotides like cUMP. Little is known about how cUMP acts in the cell. Here we found that cUMP instigates pulmonary edema during Pseudomonas aeruginosa infection of the lung.
Keywords: cyclic nucleotide monophosphate; exoenzyme Y; permeability; pneumonia; type 3 secretion system.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures





References
-
- Martin TR, Zemans RL, Ware LB, Schmidt EP, Riches DWH, Bastarache L, Calfee CS, Desai TJ, Herold S, Hough CL, Looney MR, Matthay MA, Meyer N, Parikh SM, Stevens T, Thompson BT. New insights into clinical and mechanistic heterogeneity of the acute respiratory distress syndrome: summary of the Aspen Lung Conference 2021. Am J Respir Cell Mol Biol 67: 284–308, 2022. doi:10.1165/rcmb.2022-0089WS. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- R01 HL167997/HL/NHLBI NIH HHS/United States
- HL136689/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- P01 HL066299/HL/NHLBI NIH HHS/United States
- AI104922/HHS | NIH | NIAID | Division of Microbiology and Infectious Diseases (DMID)
- R01 HL140182/HL/NHLBI NIH HHS/United States
- HL167997/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01 AI104922/AI/NIAID NIH HHS/United States
- HL148069/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01 HL148069/HL/NHLBI NIH HHS/United States
- HL140182/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- HL66299/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
LinkOut - more resources
Full Text Sources
Miscellaneous

