Specific Graft Treatment Solution Enhances Vascular Endothelial Function
- PMID: 39076175
- PMCID: PMC11269054
- DOI: 10.31083/j.rcm2311368
Specific Graft Treatment Solution Enhances Vascular Endothelial Function
Abstract
Background: Saline is still the most widely used storage and rinsing solution for vessel grafts during cardiac surgery despite knowing evidence of its negative influence on the human endothelial cell function. Aim of this study was to assess the effect of DuraGraft©, an intraoperative graft treatment solution, on human saphenous vein segments and further elaborate the vasoprotective effect on rat aortic segments in comparison to saline.
Methods: Human Saphenous vein (HSV) graft segments from patients undergoing aortocoronary bypass surgery (n = 15), were randomized to DuraGraft© (n = 15) or saline (n = 15) solution before intraoperative storage. Each segment was divided into two subsegmental parts for evaluation. These segments as well as rat aortic segments stored in DuraGraft© underwent assessment of vascular function in a multichamber isometric myograph system in comparison to Krebs-Henseleit solution (KHS), a physiologic organ buffer solution.
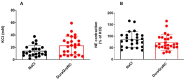
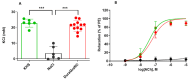
Results: Potassium-Chloride (KCL)-induced contraction depicted a tendency towards increase when treated with DuraGraft© compared to saline preservation of HSV segments (23.02 14.77 vs 14.44 9.13 mN, p = 0.0571). Vein segments preserved with DuraGraft© showed a significant improvement of endothelium-dependent vasorelaxation in response to cumulative concentrations of bradykinin compared to saline treated segments (p 0.05). Rat aortic segments stored in saline showed significantly impaired vasoconstriction (3.59 4.20, p 0.0001) and vasorelaxation when compared to KHS and DuraGraft© (p 0.0001).
Conclusions: DuraGraft© demonstrated a favorable effect on graft relaxation and contraction indicating preservation of vascular endothelial function.
Clinical trial registration number: NCT04614077.
Keywords: coronary artery bypass grafting; endothelium; myograph; preservation solution; vein graft.
Copyright: © 2022 The Author(s). Published by IMR Press.
Conflict of interest statement
The authors declare no conflict of interest. Attila Kiss is serving as one of the Guest editors of this journal. We declare that Attila Kiss had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Dragan M. Djuric and Teruo Inoue.
Figures


References
-
- Wilbring M, Tugtekin SM, Zatschler B, Ebner A, Reichenspurner H, Kappert U, et al. Preservation of endothelial vascular function of saphenous vein grafts after long-time storage with a recently developed potassium-chloride and N-acetylhistidine enriched storage solution. The Thoracic and Cardiovascular Surgeon . 2013;61:656–662. - PubMed
-
- Carrel T, Winkler B. Current trends in selection of conduits for coronary artery bypass grafting. General Thoracic and Cardiovascular Surgery . 2017;65:549–556. - PubMed
-
- Caliskan E, Sandner S, Misfeld M, Aramendi J, Salzberg SP, Choi YH, et al. A novel endothelial damage inhibitor for the treatment of vascular conduits in coronary artery bypass grafting: protocol and rationale for the European, multicentre, prospective, observational DuraGraft registry. Journal of Cardiothoracic Surgery . 2019;14:174. - PMC - PubMed
-
- Ragnarsson S, Janiec M, Modrau IS, Dreifaldt M, Ericsson A, Holmgren A, et al. No-touch saphenous vein grafts in coronary artery surgery (SWEDEGRAFT): Rationale and design of a multicenter, prospective, registry-based randomized clinical trial. American Heart Journal . 2020;224:17–24. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

